HGAL, a lymphoma prognostic biomarker, interacts with the cytoskeleton and mediates the effects of IL-6 on cell migration
- PMID: 17823310
- PMCID: PMC2234785
- DOI: 10.1182/blood-2007-04-087775
HGAL, a lymphoma prognostic biomarker, interacts with the cytoskeleton and mediates the effects of IL-6 on cell migration
Abstract
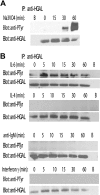
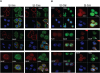
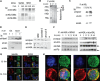
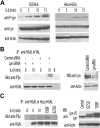
HGAL is a newly identified germinal center (GC)-specific gene whose expression by the tumor cells correlates with a favorable prognosis in patients with diffuse large B-cell and classical Hodgkin lymphomas. The function of HGAL is unknown. Previous studies demonstrated that HGAL is dispensable for GC formation, immunoglobulin gene class-switch recombination, and somatic hypermutation. Herein, we identify a role for HGAL in the regulation of cell motility. We demonstrate that IL-6 induces the phosphorylation of the C-terminal tyrosine residue of the HGAL protein via the Lyn kinase, and promotes its relocalization from the cytoplasm to podosome-like structures. Further, IL-6-induced HGAL phosphorylation increases its interaction with myosin II and is associated with inhibition of cell migration. Knockdown of endogenous HGAL ameliorates IL-6-induced inhibition of cell migration, whereas overexpression of HGAL imparts inhibitory effects of IL-6 on cell migration. Taken together, our results suggest that HGAL is involved in negative regulation of lymphocyte migration, thus constraining lymphocytes to the GC. Inhibition of lymphocyte migration might contribute to the less aggressive clinical behavior of HGAL-expressing lymphomas.
Figures


Similar articles
-
miR-155 regulates HGAL expression and increases lymphoma cell motility.Blood. 2012 Jan 12;119(2):513-20. doi: 10.1182/blood-2011-08-370536. Epub 2011 Nov 16. Blood. 2012. PMID: 22096245 Free PMC article.
-
Expression of the human germinal center-associated lymphoma (HGAL) protein, a new marker of germinal center B-cell derivation.Blood. 2005 May 15;105(10):3979-86. doi: 10.1182/blood-2004-08-3112. Epub 2005 Jan 27. Blood. 2005. PMID: 15677569 Free PMC article.
-
HGAL localization to cell membrane regulates B-cell receptor signaling.Blood. 2015 Jan 22;125(4):649-57. doi: 10.1182/blood-2014-04-571331. Epub 2014 Nov 7. Blood. 2015. PMID: 25381061 Free PMC article.
-
Regulation of BCR-dependent germinal center B-cell formation by HGAL and insight into its emerging myeloid ortholog, C1ORF150.Front Immunol. 2024 Oct 15;15:1437516. doi: 10.3389/fimmu.2024.1437516. eCollection 2024. Front Immunol. 2024. PMID: 39474423 Free PMC article. Review.
-
The BCL6 proto-oncogene: a leading role during germinal center development and lymphomagenesis.Pathol Biol (Paris). 2007 Feb;55(1):73-83. doi: 10.1016/j.patbio.2006.04.001. Epub 2006 Jul 3. Pathol Biol (Paris). 2007. PMID: 16815642 Review.
Cited by
-
The RhoA-ROCK pathway in the regulation of T and B cell responses.F1000Res. 2016 Sep 12;5:F1000 Faculty Rev-2295. doi: 10.12688/f1000research.7522.1. eCollection 2016. F1000Res. 2016. PMID: 27785353 Free PMC article. Review.
-
Interplay between HGAL and Grb2 proteins regulates B-cell receptor signaling.Blood Adv. 2019 Aug 13;3(15):2286-2297. doi: 10.1182/bloodadvances.2018016162. Blood Adv. 2019. PMID: 31362927 Free PMC article.
-
miR-155 regulates HGAL expression and increases lymphoma cell motility.Blood. 2012 Jan 12;119(2):513-20. doi: 10.1182/blood-2011-08-370536. Epub 2011 Nov 16. Blood. 2012. PMID: 22096245 Free PMC article.
-
Germinal centre protein HGAL promotes lymphoid hyperplasia and amyloidosis via BCR-mediated Syk activation.Nat Commun. 2013;4:1338. doi: 10.1038/ncomms2334. Nat Commun. 2013. PMID: 23299888 Free PMC article.
-
Germinal center-specific protein human germinal center associated lymphoma directly interacts with both myosin and actin and increases the binding of myosin to actin.FEBS J. 2011 Jun;278(11):1922-31. doi: 10.1111/j.1742-4658.2011.08109.x. Epub 2011 Apr 20. FEBS J. 2011. PMID: 21447067 Free PMC article.
References
-
- Lossos IS, Alizadeh AA, Rajapaksa R, Tibshirani R, Levy R. HGAL is a novel interleukin-4-inducible gene that strongly predicts survival in diffuse large B-cell lymphoma. Blood. 2003;101:433–440. - PubMed
-
- Burdin N, Galibert L, Garrone P, Durand I, Banchereau J, Rousset F. Inability to produce IL-6 is a functional feature of human germinal center B lymphocytes. J Immunol. 1996;156:4107–4113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

