De-phosphorylation of MyoD is linking nerve-evoked activity to fast myosin heavy chain expression in rodent adult skeletal muscle
- PMID: 17761773
- PMCID: PMC2277165
- DOI: 10.1113/jphysiol.2007.141457
De-phosphorylation of MyoD is linking nerve-evoked activity to fast myosin heavy chain expression in rodent adult skeletal muscle
Abstract
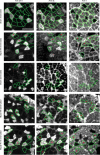
Elucidating the molecular pathways linking electrical activity to gene expression is necessary for understanding the effects of exercise on muscle. Fast muscles express higher levels of MyoD and lower levels of myogenin than slow muscles, and we have previously linked myogenin to expression of oxidative enzymes. We here report that in slow muscles, compared with fast, 6 times as much of the MyoD is in an inactive form phosphorylated at T115. In fast muscles, 10 h of slow electrical stimulation had no effect on the total MyoD protein level, but the fraction of phosphorylated MyoD was increased 4-fold. Longer stimulation also decreased the total level of MyoD mRNA and protein, while the level of myogenin protein was increased. Fast patterned stimulation did not have any of these effects. Overexpression of wild type MyoD had variable effects in active slow muscles, but increased expression of fast myosin heavy chain in denervated muscles. In normally active soleus muscles, MyoD mutated at T115 (but not at S200) increased the number of fibres containing fast myosin from 50% to 85% in mice and from 13% to 62% in rats. These data establish de-phosphorylated active MyoD as a link between the pattern of electrical activity and fast fibre type in adult muscles.
Figures
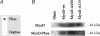

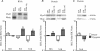


Similar articles
-
Myogenin, MyoD, and myosin heavy chain isoform expression following hindlimb suspension.Aviat Space Environ Med. 1999 May;70(5):511-6. Aviat Space Environ Med. 1999. PMID: 10332949
-
Nerve influence on myosin light chain phosphorylation in slow and fast skeletal muscles.FEBS J. 2005 Nov;272(22):5771-85. doi: 10.1111/j.1742-4658.2005.04965.x. FEBS J. 2005. PMID: 16279942
-
Activity-unrelated neural control of myogenic factors in a slow muscle.Muscle Nerve. 2006 Jan;33(1):49-60. doi: 10.1002/mus.20433. Muscle Nerve. 2006. PMID: 16184607
-
Determination of muscle contractile properties: the importance of the nerve.Acta Physiol Scand. 1998 Mar;162(3):333-41. doi: 10.1046/j.1365-201X.1998.0336e.x. Acta Physiol Scand. 1998. PMID: 9578379 Review.
-
How is muscle phenotype controlled by nerve activity?Ital J Neurol Sci. 1999 Dec;20(6):409-12. doi: 10.1007/s100720050060. Ital J Neurol Sci. 1999. PMID: 10937861 Review.
Cited by
-
MYOD mediates skeletal myogenic differentiation of human amniotic fluid stem cells and regeneration of muscle injury.Stem Cell Res Ther. 2013;4(6):147. doi: 10.1186/scrt358. Stem Cell Res Ther. 2013. PMID: 24331373 Free PMC article.
-
Postnatal muscle modification by myogenic factors modulates neuropathology and survival in an ALS mouse model.Nat Commun. 2013;4:2906. doi: 10.1038/ncomms3906. Nat Commun. 2013. PMID: 24346342 Free PMC article.
-
Myosin heavy chain mRNA isoforms are expressed in two distinct cohorts during C2C12 myogenesis.J Muscle Res Cell Motil. 2012 Mar;32(6):383-90. doi: 10.1007/s10974-011-9267-4. Epub 2011 Oct 20. J Muscle Res Cell Motil. 2012. PMID: 22012579
-
Developmental, physiologic and phylogenetic perspectives on the expression and regulation of myosin heavy chains in mammalian skeletal muscles.J Comp Physiol B. 2023 Aug;193(4):355-382. doi: 10.1007/s00360-023-01499-0. Epub 2023 Jun 5. J Comp Physiol B. 2023. PMID: 37277594 Free PMC article. Review.
-
CHCHD4-TRIAP1 regulation of innate immune signaling mediates skeletal muscle adaptation to exercise.Cell Rep. 2024 Jan 23;43(1):113626. doi: 10.1016/j.celrep.2023.113626. Epub 2023 Dec 28. Cell Rep. 2024. PMID: 38157298 Free PMC article.
References
-
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell Signal. 2002;14:649–654. - PubMed
-
- Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem. 2001;276:43524–43533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

