Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection
- PMID: 17724130
- PMCID: PMC2118701
- DOI: 10.1084/jem.20070567
Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection
Erratum in
- J Exp Med. 2007 Oct;204(10):2493
Abstract
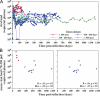
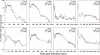
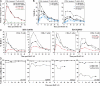
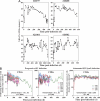
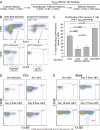
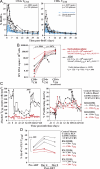
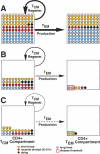
Primary simian immunodeficiency virus (SIV) infections of rhesus macaques result in the dramatic depletion of CD4(+) CCR5(+) effector-memory T (T(EM)) cells from extra-lymphoid effector sites, but in most infections, an increased rate of CD4(+) memory T cell proliferation appears to prevent collapse of effector site CD4(+) T(EM) cell populations and acute-phase AIDS. Eventually, persistent SIV replication results in chronic-phase AIDS, but the responsible mechanisms remain controversial. Here, we demonstrate that in the chronic phase of progressive SIV infection, effector site CD4(+) T(EM) cell populations manifest a slow, continuous decline, and that the degree of this depletion remains a highly significant correlate of late-onset AIDS. We further show that due to persistent immune activation, effector site CD4(+) T(EM) cells are predominantly short-lived, and that their homeostasis is strikingly dependent on the production of new CD4(+) T(EM) cells from central-memory T (T(CM)) cell precursors. The instability of effector site CD4(+) T(EM) cell populations over time was not explained by increasing destruction of these cells, but rather was attributable to progressive reduction in their production, secondary to decreasing numbers of CCR5(-) CD4(+) T(CM) cells. These data suggest that although CD4(+) T(EM) cell depletion is a proximate mechanism of immunodeficiency, the tempo of this depletion and the timing of disease onset are largely determined by destruction, failing production, and gradual decline of CD4(+) T(CM) cells.
Figures

Similar articles
-
Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection.Nature. 2005 Apr 28;434(7037):1093-7. doi: 10.1038/nature03501. Nature. 2005. PMID: 15793563
-
Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis.J Exp Med. 2009 Jul 6;206(7):1575-88. doi: 10.1084/jem.20090356. Epub 2009 Jun 22. J Exp Med. 2009. PMID: 19546246 Free PMC article.
-
Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection.J Exp Med. 2006 Nov 27;203(12):2661-72. doi: 10.1084/jem.20060134. Epub 2006 Nov 20. J Exp Med. 2006. PMID: 17116733 Free PMC article.
-
The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche.Semin Immunol. 2008 Jun;20(3):181-6. doi: 10.1016/j.smim.2008.04.002. Epub 2008 Jul 2. Semin Immunol. 2008. PMID: 18595731 Free PMC article. Review.
-
CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure.Immunol Rev. 2013 Jul;254(1):54-64. doi: 10.1111/imr.12066. Immunol Rev. 2013. PMID: 23772614 Free PMC article. Review.
Cited by
-
Analysis of the In Vivo Turnover of CD4+ T-Cell Subsets in Chronically SIV-Infected Sooty Mangabeys.PLoS One. 2016 May 26;11(5):e0156352. doi: 10.1371/journal.pone.0156352. eCollection 2016. PLoS One. 2016. PMID: 27227993 Free PMC article.
-
Nonpathogenic simian immunodeficiency virus infections.Cold Spring Harb Perspect Med. 2012 Jan;2(1):a007153. doi: 10.1101/cshperspect.a007153. Cold Spring Harb Perspect Med. 2012. PMID: 22315718 Free PMC article. Review.
-
Effect of combination antiretroviral therapy on Chinese rhesus macaques of simian immunodeficiency virus infection.AIDS Res Hum Retroviruses. 2013 Nov;29(11):1465-74. doi: 10.1089/AID.2012.0378. Epub 2013 Mar 8. AIDS Res Hum Retroviruses. 2013. PMID: 23387294 Free PMC article.
-
HIV pathogenesis: the host.Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a007005. doi: 10.1101/cshperspect.a007005. Cold Spring Harb Perspect Med. 2012. PMID: 22951442 Free PMC article. Review.
-
Measurement of proliferation and disappearance of rapid turnover cell populations in human studies using deuterium-labeled glucose.Nat Protoc. 2009;4(9):1313-27. doi: 10.1038/nprot.2009.117. Epub 2009 Aug 20. Nat Protoc. 2009. PMID: 19696750
References
-
- Pantaleo, G., C. Graziosi, J.F. Demarest, L. Butini, M. Montroni, C.H. Fox, J.M. Orenstein, D.P. Kotler, and A.S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 362:355–358. - PubMed
-
- Mellors, J.W., C.R. Rinaldo Jr., P. Gupta, R.M. White, J.A. Todd, and L.A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 272:1167–1170. - PubMed
-
- Detels, R., A. Munoz, G. McFarlane, L.A. Kingsley, J.B. Margolick, J. Giorgi, L.K. Schrager, and J.P. Phair. 1998. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 280:1497–1503. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

