The tau N279K exon 10 splicing mutation recapitulates frontotemporal dementia and parkinsonism linked to chromosome 17 tauopathy in a mouse model
- PMID: 17715352
- PMCID: PMC6672194
- DOI: 10.1523/JNEUROSCI.5492-06.2007
The tau N279K exon 10 splicing mutation recapitulates frontotemporal dementia and parkinsonism linked to chromosome 17 tauopathy in a mouse model
Abstract
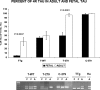
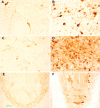
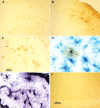
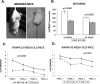
Intracellular tau deposits are characteristic of several neurodegenerative disorders called tauopathies. The tau protein regulates the stability and assembly of microtubules by binding to microtubules through three or four microtubule-binding repeats (3R and 4R). The number of microtubule-binding repeats is determined by the inclusion or exclusion of the second microtubule-binding repeat encoded by exon 10 of the TAU gene. TAU gene mutations that alter the inclusion of exon 10, and hence the 4R:3R ratio, are causal in the tauopathy frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). A mutation located in exon 10 has been identified in several FTDP-17 families that present with increased exon 10 inclusion in both mRNA and protein, parkinsonism, movement disorders, and dementia. We have engineered a human tau minigene construct that was designed to allow alternative splicing of the tau exon 10. Here we demonstrate that transgenic mice expressing human tau protein with this mutation develop neurodegeneration as result of aberrant splicing. The mice recapitulate many of the disease hallmarks that are seen in patients with this mutation, including increased tau exon 10 inclusion in both mRNA and protein, motor and behavioral deficits, and tau protein accumulation in neurons and tufted astrocytes. Furthermore, these mice present with degeneration of the nigrostriatal dopaminergic pathway, suggesting a possible mechanism for parkinsonism in FTDP-17. Additionally, activated caspase-3 immunoreactivity in both neurons and astrocytes implicates the involvement of the apoptotic pathway in the pathology of these mice.
Figures







Similar articles
-
Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).Neurogenetics. 2000 Mar;2(4):193-205. doi: 10.1007/pl00022972. Neurogenetics. 2000. PMID: 10983715 Review.
-
[The genetics of dementias. Part 1: Molecular basis of frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17)].Postepy Hig Med Dosw (Online). 2009 Jun 15;63:278-86. Postepy Hig Med Dosw (Online). 2009. PMID: 19535823 Review. Polish.
-
Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Their relevance for understanding the neurogenerative process.Ann N Y Acad Sci. 2000;920:74-83. doi: 10.1111/j.1749-6632.2000.tb06907.x. Ann N Y Acad Sci. 2000. PMID: 11193179 Review.
-
Neurodegenerative disorder FTDP-17-related tau intron 10 +16C → T mutation increases tau exon 10 splicing and causes tauopathy in transgenic mice.Am J Pathol. 2013 Jul;183(1):211-25. doi: 10.1016/j.ajpath.2013.03.015. Epub 2013 May 13. Am J Pathol. 2013. PMID: 23680655
-
[Japanese contribution to the understanding of frontotemporal dementia and parkinsonism linked to chromosome 17(FTDP-17)].No To Shinkei. 2003 Feb;55(2):107-19. No To Shinkei. 2003. PMID: 12684990 Review. Japanese.
Cited by
-
Cellular and pathological functions of tau.Nat Rev Mol Cell Biol. 2024 Nov;25(11):845-864. doi: 10.1038/s41580-024-00753-9. Epub 2024 Jul 16. Nat Rev Mol Cell Biol. 2024. PMID: 39014245 Review.
-
Frontotemporal Dementia-Associated N279K Tau Mutation Localizes at the Nuclear Compartment.Front Cell Neurosci. 2018 Jul 12;12:202. doi: 10.3389/fncel.2018.00202. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30050413 Free PMC article.
-
Pseudomonas aeruginosa exotoxin Y-mediated tau hyperphosphorylation impairs microtubule assembly in pulmonary microvascular endothelial cells.PLoS One. 2013 Sep 4;8(9):e74343. doi: 10.1371/journal.pone.0074343. eCollection 2013. PLoS One. 2013. PMID: 24023939 Free PMC article.
-
Tauopathy induced by low level expression of a human brain-derived tau fragment in mice is rescued by phenylbutyrate.Brain. 2016 Aug;139(Pt 8):2290-306. doi: 10.1093/brain/aww137. Epub 2016 Jun 12. Brain. 2016. PMID: 27297240 Free PMC article.
-
Exon-skipping antisense oligonucleotides to correct missplicing in neurogenetic diseases.Nucleic Acid Ther. 2014 Feb;24(1):69-86. doi: 10.1089/nat.2013.0461. Nucleic Acid Ther. 2014. PMID: 24506781 Free PMC article. Review.
References
-
- Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer PL. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol (Berl) 2001;101:167–173. - PubMed
-
- Arima K, Kowalska A, Hasegawa M, Mukoyama M, Watanabe R, Kawai M, Takahashi K, Iwatsubo T, Tabira T, Sunohara N. Two brothers with frontotemporal dementia and parkinsonism with an N279K mutation of the tau gene. Neurology. 2000;54:1787–1795. - PubMed
-
- Auboeuf D, Honig A, Berget SM, O'Malley BW. Coordinate regulation of transcription and splicing by steroid receptor coregulators. Science. 2002;298:416–419. - PubMed
-
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. - PubMed
-
- Berry RW, Abraha A, Lagalwar S, LaPointe N, Gamblin TC, Cryns VL, Binder LI. Inhibition of tau polymerization by its carboxy-terminal caspase cleavage fragment. Biochemistry. 2003;42:8325–8331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
