Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells
- PMID: 17698591
- PMCID: PMC2118697
- DOI: 10.1084/jem.20070841
Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells
Abstract
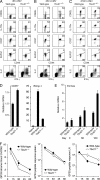
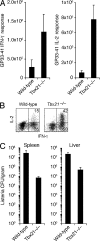
Immunity to intracellular pathogens requires dynamic balance between terminal differentiation of short-lived, cytotoxic effector CD8+ T cells and self-renewal of central-memory CD8+ T cells. We now show that T-bet represses transcription of IL-7Ralpha and drives differentiation of effector and effector-memory CD8+ T cells at the expense of central-memory cells. We also found T-bet to be overexpressed in CD8+ T cells that differentiated in the absence of CD4+ T cell help, a condition that is associated with defective central-memory formation. Finally, deletion of T-bet corrected the abnormal phenotypic and functional properties of "unhelped" memory CD8+ T cells. T-bet, thus, appears to function as a molecular switch between central- and effector-memory cell differentiation. Antagonism of T-bet may, therefore, represent a novel strategy to offset dysfunctional programming of memory CD8+ T cells.
Figures

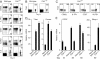

Similar articles
-
NKG2D signaling on CD8⁺ T cells represses T-bet and rescues CD4-unhelped CD8⁺ T cell memory recall but not effector responses.Nat Med. 2012 Feb 26;18(3):422-8. doi: 10.1038/nm.2683. Nat Med. 2012. PMID: 22366950 Free PMC article.
-
Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells.J Immunol. 2011 Oct 15;187(8):4068-76. doi: 10.4049/jimmunol.1002145. Epub 2011 Sep 19. J Immunol. 2011. PMID: 21930973 Free PMC article.
-
Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells.J Immunol. 2007 Jan 15;178(2):778-87. doi: 10.4049/jimmunol.178.2.778. J Immunol. 2007. PMID: 17202339
-
Transcription Factor T-bet Orchestrates Lineage Development and Function in the Immune System.Trends Immunol. 2017 Apr;38(4):287-297. doi: 10.1016/j.it.2017.02.003. Epub 2017 Mar 7. Trends Immunol. 2017. PMID: 28279590 Review.
-
Distinct regulation of effector and memory T-cell differentiation.Immunol Cell Biol. 2008 May-Jun;86(4):325-32. doi: 10.1038/icb.2008.16. Epub 2008 Mar 25. Immunol Cell Biol. 2008. PMID: 18362944 Review.
Cited by
-
Differential localization of T-bet and Eomes in CD8 T cell memory populations.J Immunol. 2013 Apr 1;190(7):3207-15. doi: 10.4049/jimmunol.1201556. Epub 2013 Mar 1. J Immunol. 2013. PMID: 23455505 Free PMC article.
-
Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade.Leukemia. 2016 Aug;30(8):1716-24. doi: 10.1038/leu.2016.84. Epub 2016 Apr 22. Leukemia. 2016. PMID: 27102208
-
Specificity and dynamics of effector and memory CD8 T cell responses in human tick-borne encephalitis virus infection.PLoS Pathog. 2015 Jan 22;11(1):e1004622. doi: 10.1371/journal.ppat.1004622. eCollection 2015 Jan. PLoS Pathog. 2015. PMID: 25611738 Free PMC article.
-
A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection.Immunity. 2009 Aug 21;31(2):309-20. doi: 10.1016/j.immuni.2009.06.019. Epub 2009 Aug 6. Immunity. 2009. PMID: 19664943 Free PMC article.
-
Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development.Nat Immunol. 2023 Mar;24(3):501-515. doi: 10.1038/s41590-023-01436-x. Epub 2023 Feb 16. Nat Immunol. 2023. PMID: 36797499
References
-
- Williams, M.A., and M.J. Bevan. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192. - PubMed
-
- Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. - PubMed
-
- Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

