Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers
- PMID: 17683545
- PMCID: PMC1976323
- DOI: 10.1186/1742-4690-4-54
Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers
Abstract
Background: The HIV-1 nucleocapsid protein (NC) is formed of two CCHC zinc fingers flanked by highly basic regions. HIV-1 NC plays key roles in virus structure and replication via its nucleic acid binding and chaperoning properties. In fact, NC controls proviral DNA synthesis by reverse transcriptase (RT), gRNA dimerization and packaging, and virion assembly.
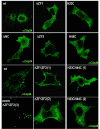
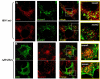
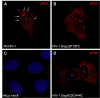
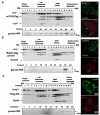
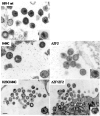
Results: We previously reported a role for the first NC zinc finger in virion structure and replication 1. To investigate the role of both NC zinc fingers in intracellular Gag trafficking, and in virion assembly, we generated series of NC zinc fingers mutations. Results show that all Zinc finger mutations have a negative impact on virion biogenesis and maturation and rendered defective the mutant viruses. The NC zinc finger mutations caused an intracellular accumulation of Gag, which was found either diffuse in the cytoplasm or at the plasma membrane but not associated with endosomal membranes as for wild type Gag. Evidences are also provided showing that the intracellular interactions between NC-mutated Gag and the gRNA were impaired.
Conclusion: These results show that Gag oligomerization mediated by gRNA-NC interactions is required for correct Gag trafficking, and assembly in HIV-1 producing cells and the release of infectious viruses.
Figures
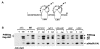
Similar articles
-
Role of the nucleocapsid domain in HIV-1 Gag oligomerization and trafficking to the plasma membrane: a fluorescence lifetime imaging microscopy investigation.J Mol Biol. 2015 Mar 27;427(6 Pt B):1480-1494. doi: 10.1016/j.jmb.2015.01.015. Epub 2015 Jan 30. J Mol Biol. 2015. PMID: 25644662
-
Nucleocapsid and matrix protein contributions to selective human immunodeficiency virus type 1 genomic RNA packaging.J Virol. 1998 Mar;72(3):1983-93. doi: 10.1128/JVI.72.3.1983-1993.1998. J Virol. 1998. PMID: 9499052 Free PMC article.
-
Mapping of nucleocapsid residues important for HIV-1 genomic RNA dimerization and packaging.Virology. 2008 Jun 5;375(2):592-610. doi: 10.1016/j.virol.2008.02.001. Epub 2008 Mar 17. Virology. 2008. PMID: 18343475
-
Mutations in the Basic Region of the Mason-Pfizer Monkey Virus Nucleocapsid Protein Affect Reverse Transcription, Genomic RNA Packaging, and the Virus Assembly Site.J Virol. 2018 Apr 27;92(10):e00106-18. doi: 10.1128/JVI.00106-18. Print 2018 May 15. J Virol. 2018. PMID: 29491167 Free PMC article.
-
Properties and functions of the nucleocapsid protein in virus assembly.RNA Biol. 2010 Nov-Dec;7(6):744-53. doi: 10.4161/rna.7.6.14065. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21157181 Free PMC article. Review.
Cited by
-
The NTD-CTD intersubunit interface plays a critical role in assembly and stabilization of the HIV-1 capsid.Retrovirology. 2013 Mar 6;10:29. doi: 10.1186/1742-4690-10-29. Retrovirology. 2013. PMID: 23497318 Free PMC article.
-
Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles.Nucleic Acids Res. 2011 Nov 1;39(20):8915-27. doi: 10.1093/nar/gkr577. Epub 2011 Jul 26. Nucleic Acids Res. 2011. PMID: 21791531 Free PMC article.
-
A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line.Retrovirology. 2009 Mar 11;6:28. doi: 10.1186/1742-4690-6-28. Retrovirology. 2009. PMID: 19284574 Free PMC article.
-
GB virus type C E2 protein inhibits human immunodeficiency virus type 1 Gag assembly by downregulating human ADP-ribosylation factor 1.Oncotarget. 2015 Dec 22;6(41):43293-309. doi: 10.18632/oncotarget.6537. Oncotarget. 2015. PMID: 26675377 Free PMC article.
-
Advances in targeting nucleocapsid-nucleic acid interactions in HIV-1 therapy.Virus Res. 2014 Nov 26;193:135-43. doi: 10.1016/j.virusres.2014.07.004. Epub 2014 Jul 12. Virus Res. 2014. PMID: 25026536 Free PMC article. Review.
References
-
- Darlix JL, Cristofari G, Rau M, Pechoux C, Berthoux L, Roques B. Nucleocapsid protein of human immunodeficiency virus as a model protein with chaperoning functions and as a target for antiviral drugs. Advances in pharmacology (San Diego, Calif. 2000;48:345–372. - PubMed
-
- Darlix JL, Lapadat-Tapolsky M, de Rocquigny H, Roques BP. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. Journal of molecular biology. 1995;254:523–537. - PubMed
-
- Demirov DG, Freed EO. Retrovirus budding. Virus research. 2004;106:87–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

