SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation
- PMID: 17681146
- PMCID: PMC2083635
- DOI: 10.1016/j.cmet.2007.07.003
SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation
Abstract
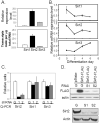
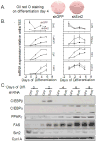
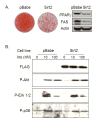
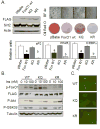
The family of mammalian Sirtuin proteins comprises seven members homologous to yeast Sir2. Here we show that SIRT2, a cytoplasmic sirtuin, is the most abundant sirtuin in adipocytes. Sirt2 expression is downregulated during preadipocyte differentiation in 3T3-L1 cells. Overexpression of SIRT2 inhibits differentiation, whereas reducing SIRT2 expression promotes adipogenesis. Both effects are accompanied by corresponding changes in the expression of PPARgamma, C/EBPalpha, and genes marking terminal adipocyte differentiation, including Glut4, aP2, and fatty acid synthase. The mechanism underlying the effects of reduced SIRT2 in 3T3-L1 adipocytes includes increased acetylation of FOXO1, with direct interaction between SIRT2 and FOXO1. This interaction enhances insulin-stimulated phosphorylation of FOXO1, which in turn regulates FOXO1 nuclear and cytosolic localization. Thus, Sirt2 acts as an important regulator of adipocyte differentiation through modulation of FOXO1 acetylation/phosphorylation and activity and may play a role in controlling adipose tissue mass and function.
Figures


Similar articles
-
Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment.J Biol Chem. 2015 Feb 13;290(7):4447-63. doi: 10.1074/jbc.M114.603977. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561724 Free PMC article.
-
SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1's repressive interaction with PPARgamma.Mol Biol Cell. 2009 Feb;20(3):801-8. doi: 10.1091/mbc.e08-06-0647. Epub 2008 Nov 26. Mol Biol Cell. 2009. PMID: 19037106 Free PMC article.
-
Fisetin induces Sirt1 expression while inhibiting early adipogenesis in 3T3-L1 cells.Biochem Biophys Res Commun. 2015 Nov 27;467(4):638-44. doi: 10.1016/j.bbrc.2015.10.094. Epub 2015 Oct 21. Biochem Biophys Res Commun. 2015. PMID: 26499075
-
Molecular mechanisms of FOXO1 in adipocyte differentiation.J Mol Endocrinol. 2019 Apr 1;62(3):R239-R253. doi: 10.1530/JME-18-0178. J Mol Endocrinol. 2019. PMID: 30780132 Review.
-
Adipose Tissue and FoxO1: Bridging Physiology and Mechanisms.Cells. 2020 Mar 31;9(4):849. doi: 10.3390/cells9040849. Cells. 2020. PMID: 32244542 Free PMC article. Review.
Cited by
-
Sirtuins and their Biological Relevance in Aging and Age-Related Diseases.Aging Dis. 2020 Jul 23;11(4):927-945. doi: 10.14336/AD.2019.0820. eCollection 2020 Jul. Aging Dis. 2020. PMID: 32765955 Free PMC article. Review.
-
Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment.J Biol Chem. 2015 Feb 13;290(7):4447-63. doi: 10.1074/jbc.M114.603977. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561724 Free PMC article.
-
Regulation of adipogenesis by cytoskeleton remodelling is facilitated by acetyltransferase MEC-17-dependent acetylation of α-tubulin.Biochem J. 2013 Feb 1;449(3):605-12. doi: 10.1042/BJ20121121. Biochem J. 2013. PMID: 23126280 Free PMC article.
-
Effects of Oxidative Stress on Mesenchymal Stem Cell Biology.Oxid Med Cell Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. Epub 2016 Jun 16. Oxid Med Cell Longev. 2016. PMID: 27413419 Free PMC article. Review.
-
Expression of the SIRT2 gene and its relationship with body size traits in Qinchuan cattle (Bos taurus).Int J Mol Sci. 2015 Jan 22;16(2):2458-71. doi: 10.3390/ijms16022458. Int J Mol Sci. 2015. PMID: 25622258 Free PMC article.
References
-
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–91. - PubMed
-
- Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–9. - PubMed
-
- Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

