Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins
- PMID: 17673908
- PMCID: PMC1952228
- DOI: 10.1038/sj.emboj.7601813
Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins
Abstract
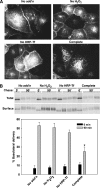
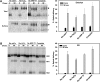
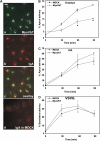
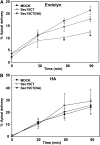
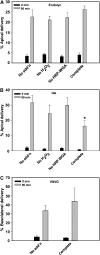
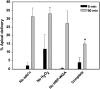
Newly synthesized basolateral markers can traverse recycling endosomes en route to the surface of Madin-Darby canine kidney cells; however, the routes used by apical proteins are less clear. Here, we functionally inactivated subsets of endocytic compartments and examined the effect on surface delivery of the basolateral marker vesicular stomatitis virus glycoprotein (VSV-G), the raft-associated apical marker influenza hemagglutinin (HA), and the non-raft-associated protein endolyn. Inactivation of transferrin-positive endosomes after internalization of horseradish peroxidase (HRP)-containing conjugates inhibited VSV-G delivery, but did not disrupt apical delivery. In contrast, inhibition of protein export from apical recycling endosomes upon expression of dominant-negative constructs of myosin Vb or Sec15 selectively perturbed apical delivery of endolyn. Ablation of apical endocytic components accessible to HRP-conjugated wheat germ agglutinin (WGA) disrupted delivery of HA but not endolyn. However, delivery of glycosylphosphatidylinositol-anchored endolyn was inhibited by >50% under these conditions, suggesting that the biosynthetic itinerary of a protein is dependent on its targeting mechanism. Our studies demonstrate that apical and basolateral proteins traverse distinct endocytic intermediates en route to the cell surface, and that multiple routes exist for delivery of newly synthesized apical proteins.
Figures

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Functional interaction between p75NTR and TrkA: the endocytic trafficking of p75NTR is driven by TrkA and regulates TrkA-mediated signalling.Biochem J. 2005 Jan 1;385(Pt 1):233-41. doi: 10.1042/BJ20041155. Biochem J. 2005. PMID: 15330756 Free PMC article.
-
Mannose receptor (MRC1) mediates uptake of dextran in macrophages via receptor-mediated endocytosis.bioRxiv [Preprint]. 2024 Aug 13:2024.08.13.607841. doi: 10.1101/2024.08.13.607841. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Dec 1;35(12):ar153. doi: 10.1091/mbc.E24-08-0355 PMID: 39211167 Free PMC article. Updated. Preprint.
-
Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD012186. doi: 10.1002/14651858.CD012186.pub2. Cochrane Database Syst Rev. 2023. PMID: 38032059 Free PMC article. Review.
Cited by
-
Rab11a is required for apical protein localisation in the intestine.Biol Open. 2014 Dec 19;4(1):86-94. doi: 10.1242/bio.20148532. Biol Open. 2014. PMID: 25527643 Free PMC article.
-
Self-organization of apical membrane protein sorting in epithelial cells.FEBS J. 2022 Feb;289(3):659-670. doi: 10.1111/febs.15882. Epub 2021 Apr 28. FEBS J. 2022. PMID: 33864720 Free PMC article.
-
Autosomal recessive polycystic kidney disease epithelial cell model reveals multiple basolateral epidermal growth factor receptor sorting pathways.Mol Biol Cell. 2010 Aug 1;21(15):2732-45. doi: 10.1091/mbc.e09-12-1059. Epub 2010 Jun 2. Mol Biol Cell. 2010. PMID: 20519437 Free PMC article.
-
Friend or Foe: The Role of the Cytoskeleton in Influenza A Virus Assembly.Viruses. 2019 Jan 10;11(1):46. doi: 10.3390/v11010046. Viruses. 2019. PMID: 30634554 Free PMC article. Review.
-
Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation.Mol Biol Cell. 2009 Nov;20(22):4652-63. doi: 10.1091/mbc.e09-02-0137. Epub 2009 Sep 23. Mol Biol Cell. 2009. PMID: 19776356 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

