The Ulp2 SUMO protease is required for cell division following termination of the DNA damage checkpoint
- PMID: 17664284
- PMCID: PMC2099214
- DOI: 10.1128/MCB.00774-07
The Ulp2 SUMO protease is required for cell division following termination of the DNA damage checkpoint
Abstract
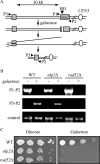
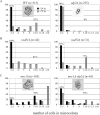
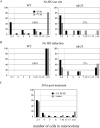
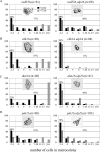
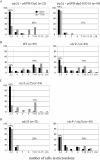
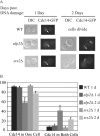
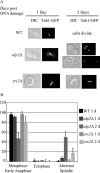
Eukaryotic genome integrity is maintained via a DNA damage checkpoint that recognizes DNA damage and halts the cell cycle at metaphase, allowing time for repair. Checkpoint signaling is eventually terminated so that the cell cycle can resume. How cells restart cell division following checkpoint termination is poorly understood. Here we show that the SUMO protease Ulp2 is required for resumption of cell division following DNA damage-induced arrest in Saccharomyces cerevisiae, although it is not required for DNA double-strand break repair. The Rad53 branch of the checkpoint pathway generates a signal countered by Ulp2 activity following DNA damage. Interestingly, unlike previously characterized adaptation mutants, ulp2Delta mutants do not show persistent Rad53 phosphorylation following DNA damage, suggesting checkpoint signaling has been terminated and no longer asserts an arrest in these cells. Using Cdc14 localization as a cell cycle indicator, we show that nearly half of cells lacking Ulp2 can escape a checkpoint-induced metaphase arrest despite their inability to divide again. Moreover, half of permanently arrested ulp2Delta cells show evidence of an aberrant mitotic spindle, suggesting that Ulp2 is required for proper spindle dynamics during cell cycle resumption following a DNA damage-induced cell cycle arrest.
Figures


Similar articles
-
Ulp2 and the DNA damage response: desumoylation enables safe passage through mitosis.Cell Cycle. 2008 Jan 1;7(1):52-6. doi: 10.4161/cc.7.1.5218. Epub 2007 Oct 28. Cell Cycle. 2008. PMID: 18196960 Review.
-
MEC3, MEC1, and DDC2 are essential components of a telomere checkpoint pathway required for cell cycle arrest during senescence in Saccharomyces cerevisiae.Mol Biol Cell. 2002 Aug;13(8):2626-38. doi: 10.1091/mbc.02-02-0012. Mol Biol Cell. 2002. PMID: 12181334 Free PMC article.
-
Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast.J Cell Biol. 2004 Feb 16;164(4):535-46. doi: 10.1083/jcb.200308100. Epub 2004 Feb 9. J Cell Biol. 2004. PMID: 14769859 Free PMC article.
-
Pph3-Psy2 is a phosphatase complex required for Rad53 dephosphorylation and replication fork restart during recovery from DNA damage.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9290-5. doi: 10.1073/pnas.0703252104. Epub 2007 May 21. Proc Natl Acad Sci U S A. 2007. PMID: 17517611 Free PMC article.
-
Phosphatases, DNA damage checkpoints and checkpoint deactivation.Cell Cycle. 2007 Dec 15;6(24):3058-64. doi: 10.4161/cc.6.24.5100. Epub 2007 Sep 20. Cell Cycle. 2007. PMID: 18075314 Review.
Cited by
-
SUMOylation and De-SUMOylation: wrestling with life's processes.J Biol Chem. 2009 Mar 27;284(13):8223-7. doi: 10.1074/jbc.R800050200. Epub 2008 Nov 13. J Biol Chem. 2009. PMID: 19008217 Free PMC article. Review.
-
SUMO Pathway Modulation of Regulatory Protein Binding at the Ribosomal DNA Locus in Saccharomyces cerevisiae.Genetics. 2016 Apr;202(4):1377-94. doi: 10.1534/genetics.116.187252. Epub 2016 Feb 2. Genetics. 2016. PMID: 26837752 Free PMC article.
-
Global analysis of SUMO chain function reveals multiple roles in chromatin regulation.J Cell Biol. 2013 Apr 1;201(1):145-63. doi: 10.1083/jcb.201210019. J Cell Biol. 2013. PMID: 23547032 Free PMC article.
-
The SUMO protease SENP6 is a direct regulator of PML nuclear bodies.Mol Biol Cell. 2011 Jan 1;22(1):78-90. doi: 10.1091/mbc.E10-06-0504. Epub 2010 Dec 9. Mol Biol Cell. 2011. PMID: 21148299 Free PMC article.
-
A high throughput mutagenic analysis of yeast sumo structure and function.PLoS Genet. 2017 Feb 6;13(2):e1006612. doi: 10.1371/journal.pgen.1006612. eCollection 2017 Feb. PLoS Genet. 2017. PMID: 28166236 Free PMC article.
References
-
- Agarwal, R., Z. Tang, H. Yu, and O. Cohen-Fix. 2003. Two distinct pathways for inhibiting Pds1 ubiquitination in response to DNA damage. J. Biol. Chem. 278:45027-45033. - PubMed
-
- Allen, J. B., Z. Zhou, W. Siede, E. C. Friedberg, and S. J. Elledge. 1994. The Sad1/Rad53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 8:2401-2415. - PubMed
-
- Bachant, J., A. Alcasabas, Y. Blat, N. Kleckner, and S. J. Elledge. 2002. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9:1169-1182. - PubMed
-
- Betting, J., and W. Seufert. 1996. A yeast Ubc9 mutant protein with temperature-sensitive in vivo function is subject to conditional proteolysis by a ubiquitin- and proteasome-dependent pathway. J. Biol. Chem. 271:25790-25796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
