Isolation of retinal progenitor and stem cells from the porcine eye
- PMID: 17653049
- PMCID: PMC2776542
Isolation of retinal progenitor and stem cells from the porcine eye
Abstract
Purpose: Retinal progenitor cells (RPCs) and retinal stem cells (RSCs) from rodents and humans have been isolated and characterized in vitro. Transplantation experiments have confirmed their potential as tools for cell replacement in retinal degenerative diseases. The pig represents an ideal pre-clinical animal model to study the impact of transplantation because of the similarity of its eye to the human eye. However, little is known about porcine RPCs and RSCs. We aimed to identify and characterize in vitro RPCs and RSCs from porcine ocular tissues.
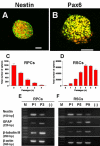
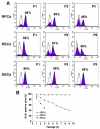
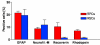
Methods: Cells from different subregions of embryonic, postnatal and adult porcine eyes were grown in suspension sphere culture in serum-free medium containing basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF). Growth curves and BrdU incorporation assays were performed to establish the proliferative capacity of isolated porcine retina-derived RPCs and ciliary epithelium (CE)-derived RSCs. Self-renewal potential was investigated by subsphere formation assays. Changes in gene expression were assayed by reverse transcription polymerase chain reaction (RT-PCR) at different passages in culture. Finally, differentiation was induced by addition of serum to the cultures and expression of markers for retinal cell types was detected by immunohistochemical staining with specific antibodies.
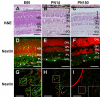
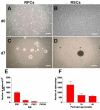
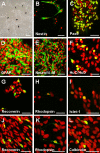
Results: Dissociated cells from embryonic retina and CE at different postnatal ages generated primary nestin- and Pax6-immunoreactive neurosphere colonies in vitro in numbers that decreased with age. Embryonic and postnatal retina-derived RPCs and young CE-derived RSCs displayed self-renewal capacity, generating secondary neurosphere colonies. However, their self-renewal and proliferation capacity gradually decreased and they became more committed to differentiated states with subsequent passages. The expansion capacity of RPCs and RSCs was higher when they were maintained in monolayer culture. Porcine RPCs and RSCs could be induced to differentiate in vitro to express markers of retinal neurons and glia.
Conclusions: Porcine retina and CE contain RPCs and RSCs which are undifferentiated, self-renewing and multipotent and which show characteristics similar to their human counterparts. Therefore, the pig could be a useful source of cells to further investigate the cell biology of RPCs and RSCs and it could be used as a non-primate large animal model for pre-clinical studies on stem cell-based approaches to regenerative medicine in the retina.
Figures
Similar articles
-
Activation of neural progenitor cells in human eyes with proliferative vitreoretinopathy.Exp Eye Res. 2012 May;98:28-36. doi: 10.1016/j.exer.2012.03.008. Epub 2012 Mar 21. Exp Eye Res. 2012. PMID: 22465407
-
Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes.Invest Ophthalmol Vis Sci. 2007 Apr;48(4):1674-82. doi: 10.1167/iovs.06-1034. Invest Ophthalmol Vis Sci. 2007. PMID: 17389499
-
In vivo reactivation of a quiescent cell population located in the ocular ciliary body of adult mammals.Exp Eye Res. 2006 Jul;83(1):153-64. doi: 10.1016/j.exer.2005.11.016. Epub 2006 Mar 23. Exp Eye Res. 2006. PMID: 16563378
-
Retinal stem cells: promising candidates for retina transplantation.Cell Tissue Res. 2008 Jan;331(1):347-57. doi: 10.1007/s00441-007-0501-8. Epub 2007 Oct 3. Cell Tissue Res. 2008. PMID: 17912553 Review.
-
Generating neuronal diversity in the retina: one for nearly all.Trends Neurosci. 2002 Jan;25(1):32-8. doi: 10.1016/s0166-2236(00)02028-2. Trends Neurosci. 2002. PMID: 11801336 Review.
Cited by
-
The parameters of the porcine eyeball.Graefes Arch Clin Exp Ophthalmol. 2011 Apr;249(4):475-82. doi: 10.1007/s00417-011-1617-9. Epub 2011 Feb 2. Graefes Arch Clin Exp Ophthalmol. 2011. PMID: 21287191 Review.
-
The Retinal Pigment Epithelium: a Convenient Source of New Photoreceptor cells?J Ophthalmic Vis Res. 2014 Jan;9(1):83-93. J Ophthalmic Vis Res. 2014. PMID: 24982737 Free PMC article.
-
The Effect of Transient Local Anti-inflammatory Treatment on the Survival of Pig Retinal Progenitor Cell Allotransplants.Transl Vis Sci Technol. 2015 Sep 22;4(5):6. doi: 10.1167/tvst.4.5.6. eCollection 2015 Sep. Transl Vis Sci Technol. 2015. PMID: 26425402 Free PMC article.
-
miR-29a regulates the proliferation and differentiation of retinal progenitors by targeting Rbm8a.Oncotarget. 2017 May 9;8(19):31993-32008. doi: 10.18632/oncotarget.16669. Oncotarget. 2017. PMID: 28404883 Free PMC article.
-
Tissue Engineering Strategies for Retina Regeneration.Appl Sci (Basel). 2021 Mar;11(5):2154. doi: 10.3390/app11052154. Epub 2021 Feb 28. Appl Sci (Basel). 2021. PMID: 35251703 Free PMC article.
References
-
- Berson EL. Retinitis pigmentosa and allied diseases. In: Albert DM, Jakobiec FA, editors. Principles and practice of ophthalmology: clinical practice. Philadelphia: Saunders; 1994. p. 1214-37.
-
- Mendes HF, van der Spuy J, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005;11:177–85. - PubMed
-
- Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 2006;25:249–76. - PubMed
-
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–72. - PubMed
-
- Ahmad I, Dooley CM, Thoreson WB, Rogers JA, Afiat S. In vitro analysis of a mammalian retinal progenitor that gives rise to neurons and glia. Brain Res. 1999;831:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
