Ins (endocytosis) and outs (exocytosis) of GLUT4 trafficking
- PMID: 17644329
- PMCID: PMC2041936
- DOI: 10.1016/j.ceb.2007.04.018
Ins (endocytosis) and outs (exocytosis) of GLUT4 trafficking
Abstract
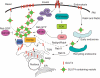
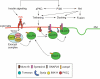
Glucose transporter 4 (GLUT4) is the major insulin-regulated glucose transporter expressed mainly in muscle and adipose tissue. GLUT4 is stored in a poorly characterized intracellular vesicular compartment and translocates to the cell surface in response to insulin stimulation resulting in an increased glucose uptake. This process is essential for the maintenance of normal glucose homeostasis and involves a complex interplay of trafficking events and intracellular signaling cascades. Recent studies have identified sortilin as an essential element for the formation of GLUT4 storage vesicles during adipogenesis and Golgi-localized gamma-ear-containing Arf-binding protein (GGA) as a key coat adaptor for the entry of newly synthesized GLUT4 into the specialized compartment. Insulin-stimulated GLUT4 translocation from this compartment to the plasma membrane appears to require the Akt/protein kinase B substrate termed AS160 (Akt substrate of 160kDa). In addition, the VPS9 domain-containing protein Gapex-5 in complex with CIP4 appears to function as a Rab31 guanylnucleotide exchange factor that is necessary for insulin-stimulated GLUT4 translocation. Here, we attempt to summarize recent advances in GLUT4 vesicle biogenesis, intracellular trafficking and membrane fusion.
Figures
Similar articles
-
The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment.Mol Endocrinol. 2007 Dec;21(12):3087-99. doi: 10.1210/me.2006-0476. Epub 2007 Aug 30. Mol Endocrinol. 2007. PMID: 17761952
-
Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes.Cell Metab. 2007 Jan;5(1):59-72. doi: 10.1016/j.cmet.2006.12.006. Cell Metab. 2007. PMID: 17189207 Free PMC article.
-
Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160.Mol Biol Cell. 2004 Oct;15(10):4406-15. doi: 10.1091/mbc.e04-04-0333. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254270 Free PMC article.
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
-
Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners.Endocrinology. 2005 Dec;146(12):5071-8. doi: 10.1210/en.2005-0850. Epub 2005 Sep 8. Endocrinology. 2005. PMID: 16150904 Review.
Cited by
-
Copper promotes the trafficking of the amyloid precursor protein.J Biol Chem. 2011 Mar 11;286(10):8252-8262. doi: 10.1074/jbc.M110.128512. Epub 2010 Dec 22. J Biol Chem. 2011. PMID: 21177866 Free PMC article.
-
Proteome Analysis of Hypoxic Glioblastoma Cells Reveals Sequential Metabolic Adaptation of One-Carbon Metabolic Pathways.Mol Cell Proteomics. 2017 Nov;16(11):1906-1921. doi: 10.1074/mcp.RA117.000154. Epub 2017 Sep 5. Mol Cell Proteomics. 2017. PMID: 28874504 Free PMC article.
-
Ankyrin-B metabolic syndrome combines age-dependent adiposity with pancreatic β cell insufficiency.J Clin Invest. 2015 Aug 3;125(8):3087-102. doi: 10.1172/JCI81317. Epub 2015 Jul 13. J Clin Invest. 2015. PMID: 26168218 Free PMC article.
-
High basal cell surface levels of fish GLUT4 are related to reduced sensitivity of insulin-induced translocation toward GGA and AS160 inhibition in adipocytes.Am J Physiol Endocrinol Metab. 2010 Feb;298(2):E329-36. doi: 10.1152/ajpendo.00547.2009. Am J Physiol Endocrinol Metab. 2010. PMID: 20075431 Free PMC article.
-
Mutant Huntingtin Affects Diabetes and Alzheimer's Markers in Human and Cell Models of Huntington's Disease.Cells. 2019 Aug 23;8(9):962. doi: 10.3390/cells8090962. Cells. 2019. PMID: 31450785 Free PMC article.
References
-
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. - PubMed
-
- Ducluzeau PH, Fletcher LM, Vidal H, Laville M, Tavare JM. Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diabetes Metab. 2002;28:85–92. - PubMed
-
- Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. - PubMed
-
- Holman GD, Sandoval IV. Moving the insulin-regulated glucose transporter GLUT4 into and out of storage. Trends Cell Biol. 2001;11:173–179. - PubMed
-
- Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

