G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility
- PMID: 17635933
- PMCID: PMC2064438
- DOI: 10.1083/jcb.200611138
G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility
Abstract
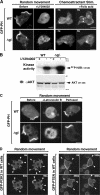
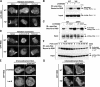
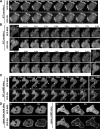
Phosphoinositide 3-kinase (PI3K)gamma and Dictyostelium PI3K are activated via G protein-coupled receptors through binding to the Gbetagamma subunit and Ras. However, the mechanistic role(s) of Gbetagamma and Ras in PI3K activation remains elusive. Furthermore, the dynamics and function of PI3K activation in the absence of extracellular stimuli have not been fully investigated. We report that gbeta null cells display PI3K and Ras activation, as well as the reciprocal localization of PI3K and PTEN, which lead to local accumulation of PI(3,4,5)P(3). Simultaneous imaging analysis reveals that in the absence of extracellular stimuli, autonomous PI3K and Ras activation occur, concurrently, at the same sites where F-actin projection emerges. The loss of PI3K binding to Ras-guanosine triphosphate abolishes this PI3K activation, whereas prevention of PI3K activity suppresses autonomous Ras activation, suggesting that PI3K and Ras form a positive feedback circuit. This circuit is associated with both random cell migration and cytokinesis and may have initially evolved to control stochastic changes in the cytoskeleton.
Figures

Similar articles
-
Finding the way: directional sensing and cell polarization through Ras signalling.Novartis Found Symp. 2005;269:73-87; discussion 87-91, 223-30. Novartis Found Symp. 2005. PMID: 16355536
-
Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement.J Cell Biol. 2004 Nov 8;167(3):505-18. doi: 10.1083/jcb.200406177. J Cell Biol. 2004. PMID: 15534002 Free PMC article.
-
Ras activation and symmetry breaking during Dictyostelium chemotaxis.J Cell Sci. 2013 Oct 1;126(Pt 19):4502-13. doi: 10.1242/jcs.132340. Epub 2013 Jul 25. J Cell Sci. 2013. PMID: 23886948
-
Regulation of chemotaxis by the orchestrated activation of Ras, PI3K, and TOR.Eur J Cell Biol. 2006 Sep;85(9-10):873-95. doi: 10.1016/j.ejcb.2006.04.007. Epub 2006 Jun 5. Eur J Cell Biol. 2006. PMID: 16740339 Review.
-
The regulation of cell motility and chemotaxis by phospholipid signaling.J Cell Sci. 2008 Mar 1;121(Pt 5):551-9. doi: 10.1242/jcs.023333. J Cell Sci. 2008. PMID: 18287584 Free PMC article. Review.
Cited by
-
Molecular basis of dynamic relocalization of Dictyostelium myosin IB.J Biol Chem. 2012 Apr 27;287(18):14923-36. doi: 10.1074/jbc.M111.318667. Epub 2012 Feb 24. J Biol Chem. 2012. PMID: 22367211 Free PMC article.
-
PIP5K-Ras bistability initiates plasma membrane symmetry breaking to regulate cell polarity and migration.bioRxiv [Preprint]. 2024 Sep 15:2024.09.15.613115. doi: 10.1101/2024.09.15.613115. bioRxiv. 2024. PMID: 39314378 Free PMC article. Preprint.
-
Involvement of the cytoskeleton in controlling leading-edge function during chemotaxis.Mol Biol Cell. 2010 Jun 1;21(11):1810-24. doi: 10.1091/mbc.e10-01-0009. Epub 2010 Apr 7. Mol Biol Cell. 2010. PMID: 20375144 Free PMC article.
-
Open access microfluidic device for the study of cell migration during chemotaxis.Integr Biol (Camb). 2010 Nov;2(11-12):648-58. doi: 10.1039/c0ib00110d. Epub 2010 Oct 15. Integr Biol (Camb). 2010. PMID: 20949221 Free PMC article.
-
Intracellular encoding of spatiotemporal guidance cues in a self-organizing signaling system for chemotaxis in Dictyostelium cells.Biophys J. 2013 Nov 5;105(9):2199-209. doi: 10.1016/j.bpj.2013.09.024. Biophys J. 2013. PMID: 24209866 Free PMC article.
References
-
- Comer, F.I., C.K. Lippincott, J.J. Masbad, and C.A. Parent. 2005. The PI3K-mediated activation of CRAC independently regulates adenylyl cyclase activation and chemotaxis. Curr. Biol. 15:134–139. - PubMed
-
- Condeelis, J., and J.E. Segall. 2003. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 3:921–930. - PubMed
-
- Engelman, J.A., J. Luo, and L.C. Cantley. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7:606–619. - PubMed
-
- Funamoto, S., R. Meili, S. Lee, L. Parry, and R. Firtel. 2002. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 109:611–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM080370-03/GM/NIGMS NIH HHS/United States
- R01 GM037830/GM/NIGMS NIH HHS/United States
- R01 GM080370/GM/NIGMS NIH HHS/United States
- GM037830/GM/NIGMS NIH HHS/United States
- R01 GM034933/GM/NIGMS NIH HHS/United States
- GM28007/GM/NIGMS NIH HHS/United States
- R01 GM080370-02/GM/NIGMS NIH HHS/United States
- R01 GM071920/GM/NIGMS NIH HHS/United States
- R01 GM028007/GM/NIGMS NIH HHS/United States
- GM34933/GM/NIGMS NIH HHS/United States
- R01 GM080370-01A2/GM/NIGMS NIH HHS/United States
- R37 GM028007/GM/NIGMS NIH HHS/United States
- GM071920/GM/NIGMS NIH HHS/United States
- R01 GM080370-04/GM/NIGMS NIH HHS/United States
- GM24279/GM/NIGMS NIH HHS/United States
- R01 GM024279/GM/NIGMS NIH HHS/United States
- GM06847/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

