Daily versus as-needed inhaled corticosteroid for mild persistent asthma (The Helsinki early intervention childhood asthma study)
- PMID: 17634183
- PMCID: PMC2532957
- DOI: 10.1136/adc.2007.116632
Daily versus as-needed inhaled corticosteroid for mild persistent asthma (The Helsinki early intervention childhood asthma study)
Abstract
Objective: To compare the effect of inhaled budesonide given daily or as-needed on mild persistent childhood asthma. Patients, design and
Interventions: 176 children aged 5-10 years with newly detected asthma were randomly assigned to three treatment groups: (1) continuous budesonide (400 microg twice daily for 1 month, 200 microg twice daily for months 2-6, 100 microg twice daily for months 7-18); (2) budesonide, identical treatment to group 1 during months 1-6, then budesonide for exacerbations as needed for months 7-18; and (3) disodium cromoglycate (DSCG) 10 mg three times daily for months 1-18. Exacerbations were treated with budesonide 400 microg twice daily for 2 weeks.
Main outcome measures: Lung function, the number of exacerbations and growth.
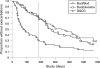
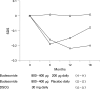
Results: Compared with DSCG the initial regular budesonide treatment resulted in a significantly improved lung function, fewer exacerbations and a small but significant decline in growth velocity. After 18 months, however, the lung function improvements did not differ between the groups. During months 7-18, patients receiving continuous budesonide treatment had significantly fewer exacerbations (mean 0.97), compared with 1.69 in group 2 and 1.58 in group 3. The number of asthma-free days did not differ between regular and intermittent budesonide treatment. Growth velocity was normalised during continuous low-dose budesonide and budesonide therapy given as needed. The latter was associated with catch-up growth.
Conclusions: Regular use of budesonide afforded better asthma control but had a more systemic effect than did use of budesonide as needed. The dose of ICS could be reduced as soon as asthma is controlled. Some children do not seem to need continuous ICS treatment.
Conflict of interest statement
Figures


Comment in
-
Do the benefits of daily inhaled steroid treatment of mild asthma outweigh the risks?Arch Dis Child. 2008 Aug;93(8):644-5. doi: 10.1136/adc.2007.135145. Arch Dis Child. 2008. PMID: 18644931 No abstract available.
-
Regular use of inhaled corticosteroids controls symptoms of mild persistent asthma, but with growth effect.J Pediatr. 2009 Jan;154(1):150. doi: 10.1016/j.jpeds.2008.10.024. J Pediatr. 2009. PMID: 19187746 No abstract available.
Similar articles
-
Bone mineral density in children treated with daily or periodical inhaled budesonide: the Helsinki Early Intervention Childhood Asthma study.Pediatr Res. 2010 Aug;68(2):169-73. doi: 10.1203/PDR.0b013e3181e69e36. Pediatr Res. 2010. PMID: 20485203 Clinical Trial.
-
Skin thickness in children treated with daily or periodical inhaled budesonide for mild persistent asthma. The Helsinki early intervention childhood asthma study.Pediatr Res. 2010 Feb;67(2):221-5. doi: 10.1203/PDR.0b013e3181c6e574. Pediatr Res. 2010. PMID: 19858777 Clinical Trial.
-
Once-daily dosing with budesonide/formoterol compared with twice-daily budesonide/formoterol and once-daily budesonide in adults with mild to moderate asthma.Respir Med. 2006 Dec;100(12):2151-9. doi: 10.1016/j.rmed.2006.03.016. Epub 2006 May 15. Respir Med. 2006. PMID: 16701989 Clinical Trial.
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Cochrane Database Syst Rev. 2014 Jul 17;2014(7):CD009471. doi: 10.1002/14651858.CD009471.pub2. Cochrane Database Syst Rev. 2014. PMID: 25030198 Free PMC article. Review.
-
Treatment of persistent asthma with Symbicort (budesonide/formoterol inhalation aerosol): an inhaled corticosteroid and long-acting beta2-adrenergic agonist in one pressurized metered-dose inhaler.J Asthma. 2010 May;47(4):447-59. doi: 10.3109/02770901003725684. J Asthma. 2010. PMID: 20528601 Review.
Cited by
-
2022 Year in Review: Pediatric Asthma.Respir Care. 2023 Oct;68(10):1430-1437. doi: 10.4187/respcare.10913. Epub 2023 May 9. Respir Care. 2023. PMID: 37160339 Free PMC article. Review.
-
Intermittent inhaled corticosteroid therapy versus placebo for persistent asthma in children and adults.Cochrane Database Syst Rev. 2015 Jul 22;2015(7):CD011032. doi: 10.1002/14651858.CD011032.pub2. Cochrane Database Syst Rev. 2015. PMID: 26197430 Free PMC article. Review.
-
Budesonide Attains Its Wide Clinical Profile by Alternative Kinetics.Pharmaceuticals (Basel). 2024 Apr 15;17(4):503. doi: 10.3390/ph17040503. Pharmaceuticals (Basel). 2024. PMID: 38675463 Free PMC article. Review.
-
On-demand intermittent beclomethasone is effective for mild asthma in Brazil.Clin Transl Allergy. 2018 Mar 5;8:7. doi: 10.1186/s13601-018-0192-0. eCollection 2018. Clin Transl Allergy. 2018. PMID: 29515802 Free PMC article.
-
Cost-utility analysis of daily versus intermittent inhaled corticosteroids in mild-persistent asthma.Pediatr Pulmonol. 2015 Aug;50(8):735-46. doi: 10.1002/ppul.23073. Epub 2014 Jun 25. Pediatr Pulmonol. 2015. PMID: 24965279 Free PMC article. Review.
References
-
- Åberg N, Engström I. Natural history of allergic diseases in childen. Acta Paediatr Scand 1990;79:206–11 - PubMed
-
- Haahtela T, Järvinen M, Kava T, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med 1994;331:700–705 - PubMed
-
- Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373–81 - PubMed
-
- Zeiger RS, Dawson C, Weiss S. Relationship between duration of asthma and asthma severity among children in the Childhood Asthma Management Program. J Allergy Clin Immunol 1999;103:376–87 - PubMed
-
- van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, et al. Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547–54 - PubMed
