The effects of montelukast on tissue inflammatory and bone marrow responses in murine experimental allergic rhinitis: interaction with interleukin-5 deficiency
- PMID: 17627772
- PMCID: PMC2266019
- DOI: 10.1111/j.1365-2567.2007.02664.x
The effects of montelukast on tissue inflammatory and bone marrow responses in murine experimental allergic rhinitis: interaction with interleukin-5 deficiency
Abstract
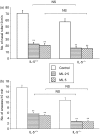
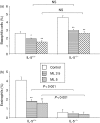
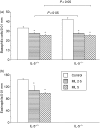
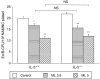
The cysteinyl leukotrienes (cysLTs) are potent lipid mediators in allergic disease, acting through the receptors, cysLT1R and cysLTR2, and are produced by eosinophils derived from eosinophil/basophil (Eo/B) bone marrow (BM) progenitors. We have demonstrated the suppressive effects of either interleukin-5 (IL-5) deficiency or montelukast on eosinophil recruitment in murine allergic rhinitis, but neither of them fully abrogated the symptoms caused by residual inflammation and cytokine redundancy in eliciting BM Eo/B responses. We hypothesized that IL-5 deficiency and montelukast act synergistically to suppress tissue inflammatory and BM responses. Our objective was to investigate the effects of the cysLT1R antagonist, montelukast, on in vivo tissue inflammatory and BM responses in murine experimental allergic rhinitis with or without IL-5 deficiency. Three groups of age-matched BALB/c mice with or without IL-5 deficiency were tested: controls (ovalbumin sensitization and challenge, placebo treatment) and two montelukast-treated groups (2.5 mg/kg or 5 mg/kg). Nasal symptoms, BM and nasal mucosal eosinophils, basophils, and BM Eo/B colony-forming units (CFU) were evaluated. Montelukast decreased nasal symptoms in a dose-dependent manner, and significantly decreased the number of eosinophils in both BM and nasal tissue in IL-5-replete mice compared to controls. In IL-5-deficient mice, in which eosinophilia was absent, montelukast significantly decreased both nasal symptoms and basophils in BM and nasal mucosal tissue, and lowered IL-5-responsive Eo/B-CFU ex vivo, compared to controls. The addition of cysLT1R blockade to IL-5 deficiency more fully attenuates symptoms and upper airway inflammation than either factor alone, providing evidence of systemic, BM mechanisms in allergic rhinitis.
Figures

Similar articles
-
Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency.J Immunol. 2002 Mar 15;168(6):3017-23. doi: 10.4049/jimmunol.168.6.3017. J Immunol. 2002. PMID: 11884474
-
Effects of a cysteinyl leukotriene receptor antagonist on eosinophil recruitment in experimental allergic rhinitis.Immunology. 2004 Oct;113(2):246-52. doi: 10.1111/j.1365-2567.2004.01944.x. Immunology. 2004. PMID: 15379985 Free PMC article.
-
Comparison of the efficacy of KOB03, ketotifen, and montelukast in an experimental mouse model of allergic rhinitis.Int Immunopharmacol. 2013 Jun;16(2):254-60. doi: 10.1016/j.intimp.2013.04.011. Epub 2013 May 1. Int Immunopharmacol. 2013. PMID: 23643816
-
Montelukast in the treatment of allergic rhinitis: an evidence-based review.Drugs. 2007;67(6):887-901. doi: 10.2165/00003495-200767060-00005. Drugs. 2007. PMID: 17428106 Review.
-
A review of montelukast in the treatment of asthma and allergic rhinitis.Expert Opin Pharmacother. 2004 Mar;5(3):679-86. doi: 10.1517/14656566.5.3.679. Expert Opin Pharmacother. 2004. PMID: 15013935 Review.
Cited by
-
Zinc and iron complexes of oleanolic acid, (OA) attenuate allergic airway inflammation in rats.Inflammopharmacology. 2019 Dec;27(6):1179-1192. doi: 10.1007/s10787-019-00597-2. Epub 2019 May 8. Inflammopharmacology. 2019. PMID: 31069605
References
-
- Salib RJ, Drake-Lee A, Howarth PH. Allergic rhinitis: past, present and the future. Clin Otolaryngol Allied Sci. 2003;28:291–303. - PubMed
-
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. - PubMed
-
- Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115:953–9. - PubMed
-
- Kroegel C, Costabel U. Immune functions of constitutive pulmonary cells: the salt in the soup. Eur Respir J. 1994;7:2106–7. - PubMed
-
- Gauvreau GM, Denburg JA. Hemopoietic progenitors. the role of eosinophil/basophil progenitors in allergic airway inflammation. Expert Rev Clin Immunol. 2005;1:87–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

