Role of endocytosis and low pH in murine hepatitis virus strain A59 cell entry
- PMID: 17626088
- PMCID: PMC2045462
- DOI: 10.1128/JVI.00725-07
Role of endocytosis and low pH in murine hepatitis virus strain A59 cell entry
Abstract
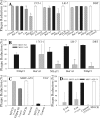
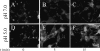
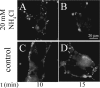
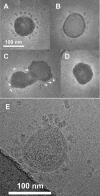
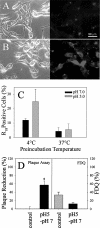
Infection by the coronavirus mouse hepatitis virus strain A59 (MHV-A59) requires the release of the viral genome by fusion with the respective target membrane of the host cell. Fusion is mediated by the viral S protein. Here, the entry pathway of MHV-A59 into murine fibroblast cells was studied by independent approaches. Infection of cells assessed by plaque reduction assay was strongly inhibited by lysosomotropic compounds and substances that interfere with clathrin-dependent endocytosis, suggesting that MHV-A59 is taken up via endocytosis and delivered to acidic endosomal compartments. Infection was only slightly reduced in the presence of substances inhibiting proteases of endosomal compartments, precluding that the endocytic uptake is required to activate the fusion potential of the S protein by its cleavage. Fluorescence confocal microscopy of labeled MHV-A59 confirmed that virus is taken up via endocytosis. Bright labeling of intracellular compartments suggests their fusion with the viral envelope. No fusion with the plasma membrane was observed at neutral pH conditions. However, when virus was bound to cells and the pH was lowered to 5.0, we observed a strong labeling of the plasma membrane. Electron microscopy revealed low pH triggered conformational alterations of the S ectodomain. Very likely, these alterations are irreversible because low-pH treatment of viruses in the absence of target membranes caused an irreversible loss of the fusion activity. The results imply that endocytosis plays a major role in MHV-A59 infection and the acidic pH of the endosomal compartment triggers a conformational change of the S protein mediating fusion.
Figures

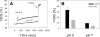
Similar articles
-
Identification of H209 as Essential for pH 8-Triggered Receptor-Independent Syncytium Formation by S Protein of Mouse Hepatitis Virus A59.J Virol. 2018 May 14;92(11):e00209-18. doi: 10.1128/JVI.00209-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514915 Free PMC article.
-
Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry.J Virol. 2006 Jun;80(12):5768-76. doi: 10.1128/JVI.00442-06. J Virol. 2006. PMID: 16731916 Free PMC article.
-
Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37 degrees C either by soluble murine CEACAM1 receptors or by pH 8.J Virol. 2003 Jan;77(2):830-40. doi: 10.1128/jvi.77.2.830-840.2003. J Virol. 2003. PMID: 12502799 Free PMC article.
-
MHVR-independent cell-cell spread of mouse hepatitis virus infection requires neutral pH fusion.Adv Exp Med Biol. 1995;380:351-7. doi: 10.1007/978-1-4615-1899-0_57. Adv Exp Med Biol. 1995. PMID: 8830507 Review.
-
[Cell entry mechanisms of coronaviruses].Uirusu. 2009 Dec;59(2):215-22. doi: 10.2222/jsv.59.215. Uirusu. 2009. PMID: 20218330 Review. Japanese.
Cited by
-
Structure, Function, and Evolution of Coronavirus Spike Proteins.Annu Rev Virol. 2016 Sep 29;3(1):237-261. doi: 10.1146/annurev-virology-110615-042301. Epub 2016 Aug 25. Annu Rev Virol. 2016. PMID: 27578435 Free PMC article. Review.
-
Mouse hepatitis virus type 2 enters cells through a clathrin-mediated endocytic pathway independent of Eps15.J Virol. 2008 Aug;82(16):8112-23. doi: 10.1128/JVI.00837-08. Epub 2008 Jun 11. J Virol. 2008. PMID: 18550663 Free PMC article.
-
ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells.J Virol. 2015 Apr;89(8):4434-48. doi: 10.1128/JVI.03274-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653449 Free PMC article.
-
Spike Glycoprotein Is Central to Coronavirus Pathogenesis-Parallel Between m-CoV and SARS-CoV-2.Ann Neurosci. 2021 Jul;28(3-4):201-218. doi: 10.1177/09727531211023755. Epub 2021 Oct 12. Ann Neurosci. 2021. PMID: 35341224 Free PMC article. Review.
-
Differential role for low pH and cathepsin-mediated cleavage of the viral spike protein during entry of serotype II feline coronaviruses.Vet Microbiol. 2008 Dec 10;132(3-4):235-48. doi: 10.1016/j.vetmic.2008.05.019. Epub 2008 May 29. Vet Microbiol. 2008. PMID: 18606506 Free PMC article.
References
-
- Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. - PubMed
-
- Blumenthal, R., A. Bali-Puri, A. Walter, D. Covell, and O. Eidelman. 1987. pH-dependent fusion of vesicular stomatitis virus with Vero cells: measurement by dequenching of octadecyl rhodamine fluorescence. J. Biol. Chem. 262:13614-13619. - PubMed
-
- Blumenthal, R., S. A. Gallo, M. Viard, Y. Raviv, and A. Puri. 2002. Fluorescent lipid probes in the study of viral membrane fusion. Chem. Phys. Lipids 116:39-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

