Dynamic remodelling of human 7SK snRNP controls the nuclear level of active P-TEFb
- PMID: 17611602
- PMCID: PMC1949012
- DOI: 10.1038/sj.emboj.7601783
Dynamic remodelling of human 7SK snRNP controls the nuclear level of active P-TEFb
Abstract
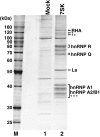
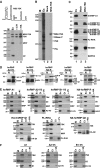
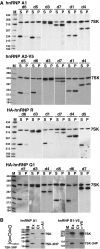
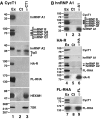
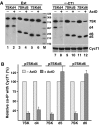
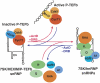
The 7SK small nuclear RNA (snRNA) regulates RNA polymerase II transcription elongation by controlling the protein kinase activity of the positive transcription elongation factor b (P-TEFb). In cooperation with HEXIM1, the 7SK snRNA sequesters P-TEFb into the kinase-inactive 7SK/HEXIM1/P-TEFb small nuclear ribonucleoprotein (snRNP), and thereby, controls the nuclear level of active P-TEFb. Here, we report that a fraction of HeLa 7SK snRNA that is not involved in 7SK/HEXIM1/P-TEFb formation, specifically interacts with RNA helicase A (RHA), heterogeneous nuclear ribonucleoprotein A1 (hnRNP), A2/B1, R and Q proteins. Inhibition of cellular transcription induces disassembly of 7SK/HEXIM1/P-TEFb and at the same time, increases the level of 7SK snRNPs containing RHA, hnRNP A1, A2/B1, R and Q. Removal of transcription inhibitors restores the original levels of the 7SK/HEXIM1/P-TEFb and '7SK/hnRNP' complexes. 7SK/HEXIM1/P-TEFb snRNPs containing mutant 7SK RNAs lacking the capacity for binding hnRNP A1, A2, R and Q are resistant to stress-induced disassembly, indicating that recruitment of the novel 7SK snRNP proteins is essential for disruption of 7SK/HEXIM1/P-TEFb. Thus, we propose that the nuclear level of active P-TEFb is controlled by dynamic and reversible remodelling of 7SK snRNP.
Figures


Similar articles
-
The transcription-dependent dissociation of P-TEFb-HEXIM1-7SK RNA relies upon formation of hnRNP-7SK RNA complexes.Mol Cell Biol. 2007 Oct;27(20):6996-7006. doi: 10.1128/MCB.00975-07. Epub 2007 Aug 20. Mol Cell Biol. 2007. PMID: 17709395 Free PMC article.
-
Regulation of polymerase II transcription by 7SK snRNA: two distinct RNA elements direct P-TEFb and HEXIM1 binding.Mol Cell Biol. 2006 Jan;26(2):630-42. doi: 10.1128/MCB.26.2.630-642.2006. Mol Cell Biol. 2006. PMID: 16382153 Free PMC article.
-
siRNA depletion of 7SK snRNA induces apoptosis but does not affect expression of the HIV-1 LTR or P-TEFb-dependent cellular genes.J Cell Physiol. 2005 Dec;205(3):463-70. doi: 10.1002/jcp.20528. J Cell Physiol. 2005. PMID: 16152622
-
Cracking the control of RNA polymerase II elongation by 7SK snRNP and P-TEFb.Nucleic Acids Res. 2016 Sep 19;44(16):7527-39. doi: 10.1093/nar/gkw585. Epub 2016 Jul 1. Nucleic Acids Res. 2016. PMID: 27369380 Free PMC article. Review.
-
RNA-driven cyclin-dependent kinase regulation: when CDK9/cyclin T subunits of P-TEFb meet their ribonucleoprotein partners.Biotechnol J. 2008 Aug;3(8):1022-32. doi: 10.1002/biot.200800104. Biotechnol J. 2008. PMID: 18655042 Review.
Cited by
-
RNA polymerase II transcription elongation control.Chem Rev. 2013 Nov 13;113(11):8583-603. doi: 10.1021/cr400105n. Epub 2013 Aug 6. Chem Rev. 2013. PMID: 23919563 Free PMC article. Review. No abstract available.
-
HEXIM1 targets a repeated GAUC motif in the riboregulator of transcription 7SK and promotes base pair rearrangements.Nucleic Acids Res. 2010 Nov;38(21):7749-63. doi: 10.1093/nar/gkq660. Epub 2010 Jul 31. Nucleic Acids Res. 2010. PMID: 20675720 Free PMC article.
-
RNA-mediated displacement of an inhibitory snRNP complex activates transcription elongation.Nat Struct Mol Biol. 2010 Jul;17(7):815-21. doi: 10.1038/nsmb.1827. Epub 2010 Jun 20. Nat Struct Mol Biol. 2010. PMID: 20562857 Free PMC article.
-
A capping-independent function of MePCE in stabilizing 7SK snRNA and facilitating the assembly of 7SK snRNP.Nucleic Acids Res. 2010 Jan;38(2):360-9. doi: 10.1093/nar/gkp977. Epub 2009 Nov 11. Nucleic Acids Res. 2010. PMID: 19906723 Free PMC article.
-
Discovery of a large-scale, cell-state-responsive allosteric switch in the 7SK RNA using DANCE-MaP.Mol Cell. 2022 May 5;82(9):1708-1723.e10. doi: 10.1016/j.molcel.2022.02.009. Epub 2022 Mar 22. Mol Cell. 2022. PMID: 35320755 Free PMC article.
References
-
- Aguilera A (2005) Cotranscriptional mRNP assembly: from the DNA to the nuclear pore. Curr Opin Cell Biol 17: 242–250 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

