Mechanisms of disease: new therapeutic strategies for Alzheimer's disease--targeting APP processing in lipid rafts
- PMID: 17611486
- PMCID: PMC2880530
- DOI: 10.1038/ncpneuro0549
Mechanisms of disease: new therapeutic strategies for Alzheimer's disease--targeting APP processing in lipid rafts
Abstract
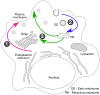
Alzheimer's disease (AD) is the most common cause of age-related dementia. Pathologically, AD is characterized by the deposition in the brain of amyloid-beta peptides derived from proteolysis of amyloid precursor protein (APP) by beta-site APP cleaving enzyme 1 (BACE1) and gamma-secretase. A growing body of evidence implicates cholesterol and cholesterol-rich membrane microdomains in amyloidogenic processing of APP. Here, we review recent findings regarding the association of BACE1, gamma-secretase and APP in lipid rafts, and discuss potential therapeutic strategies for AD that are based on knowledge gleaned from the membrane environment that fosters APP processing.
Figures

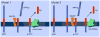
Similar articles
-
Targeting ADAM10 to lipid rafts in neuroblastoma SH-SY5Y cells impairs amyloidogenic processing of the amyloid precursor protein.Brain Res. 2009 Nov 3;1296:203-15. doi: 10.1016/j.brainres.2009.07.105. Epub 2009 Aug 11. Brain Res. 2009. PMID: 19679113
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Biophysical alterations in lipid rafts from human cerebral cortex associate with increased BACE1/AβPP interaction in early stages of Alzheimer's disease.J Alzheimers Dis. 2015;43(4):1185-98. doi: 10.3233/JAD-141146. J Alzheimers Dis. 2015. PMID: 25147112
-
Membrane rafts in Alzheimer's disease beta-amyloid production.Biochim Biophys Acta. 2010 Aug;1801(8):860-7. doi: 10.1016/j.bbalip.2010.03.007. Epub 2010 Mar 18. Biochim Biophys Acta. 2010. PMID: 20303415 Free PMC article. Review.
-
Β-site APP-cleaving enzyme 1 trafficking and Alzheimer's disease pathogenesis.J Neurochem. 2012 Mar;120(6):869-80. doi: 10.1111/j.1471-4159.2011.07623.x. Epub 2012 Jan 23. J Neurochem. 2012. PMID: 22171895 Review.
Cited by
-
High glucose-mediated PICALM and mTORC1 modulate processing of amyloid precursor protein via endosomal abnormalities.Br J Pharmacol. 2020 Aug;177(16):3828-3847. doi: 10.1111/bph.15131. Epub 2020 Jul 14. Br J Pharmacol. 2020. PMID: 32436237 Free PMC article.
-
Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing.Neurochem Int. 2011 Feb;58(3):321-9. doi: 10.1016/j.neuint.2010.12.004. Epub 2010 Dec 22. Neurochem Int. 2011. PMID: 21184792 Free PMC article.
-
Lipid rafts participate in aberrant degradative autophagic-lysosomal pathway of amyloid-beta peptide in Alzheimer's disease.Neural Regen Res. 2014 Jan 1;9(1):92-100. doi: 10.4103/1673-5374.125335. Neural Regen Res. 2014. PMID: 25206748 Free PMC article. Review.
-
Loss of cleavage at β'-site contributes to apparent increase in β-amyloid peptide (Aβ) secretion by β-secretase (BACE1)-glycosylphosphatidylinositol (GPI) processing of amyloid precursor protein.J Biol Chem. 2011 Jul 22;286(29):26166-77. doi: 10.1074/jbc.M111.260471. Epub 2011 Jun 3. J Biol Chem. 2011. PMID: 21642424 Free PMC article.
-
Links between copper and cholesterol in Alzheimer's disease.Front Physiol. 2013 May 16;4:111. doi: 10.3389/fphys.2013.00111. eCollection 2013. Front Physiol. 2013. PMID: 23720634 Free PMC article.
References
-
- Alzheimer A. On a peculiar disease of the cerebral cortex [German] Allgemeine Zeitschrift für Psychiatrie und Psychisch-Gerichtliche Medizin. 1907;64:146–148.
-
- Vassar R. BACE1: the β-secretase enzyme in Alzheimer’s disease. J Mol Neurosci. 2004;23:105–114. - PubMed
-
- Allinson TM, et al. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. - PubMed
-
- Iwatsubo T. The γ-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004;14:379–383. - PubMed
-
- Li Q, et al. Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE 1. J Biol Chem. 2004;279:10542–10550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
