Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival
- PMID: 17609386
- PMCID: PMC1906726
- DOI: 10.1073/pnas.0700490104
Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival
Abstract
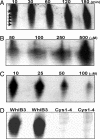
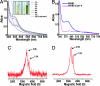
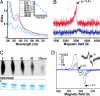
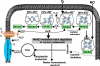
A fundamental challenge in the redox biology of Mycobacterium tuberculosis (Mtb) is to understand the mechanisms involved in sensing redox signals such as oxygen (O2), nitric oxide (NO), and nutrient depletion, which are thought to play a crucial role in persistence. Here we show that Mtb WhiB3 responds to the dormancy signals NO and O2 through its iron-sulfur (Fe-S) cluster. To functionally assemble the WhiB3 Fe-S cluster, we identified and characterized the Mtb cysteine desulfurase (IscS; Rv3025c) and developed a native enzymatic reconstitution system for assembling Fe-S clusters in Mtb. EPR and UV-visible spectroscopy analysis of reduced WhiB3 is consistent with a one-electron reduction of EPR silent [4Fe-4S]2+ to EPR visible [4Fe-4S]+. Atmospheric O2 gradually degrades the WhiB3 [4Fe-4S]2+ cluster to generate a [3Fe-4S]+ intermediate. Furthermore, EPR analysis demonstrates that NO forms a protein-bound dinitrosyl-iron-dithiol complex with the Fe-S cluster, indicating that NO specifically targets the WhiB3 Fe-S cluster. Our data suggest that the mechanism of WhiB3 4Fe-4S cluster degradation is similar to that of fumarate nitrate regulator. Importantly, Mtb DeltawhiB3 shows enhanced growth on acetate medium, but a growth defect on media containing glucose, pyruvate, succinate, or fumarate as the sole carbon source. Our results implicate WhiB3 in metabolic switching and in sensing the physiologically relevant host signaling molecules NO and O2 through its [4Fe-4S] cluster. Taken together, our results suggest that WhiB3 is an intracellular redox sensor that integrates environmental redox signals with core intermediary metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mycobacterium tuberculosis SufR responds to nitric oxide via its 4Fe-4S cluster and regulates Fe-S cluster biogenesis for persistence in mice.Redox Biol. 2021 Oct;46:102062. doi: 10.1016/j.redox.2021.102062. Epub 2021 Jul 2. Redox Biol. 2021. PMID: 34392160 Free PMC article.
-
Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide.Acc Chem Res. 2014 Oct 21;47(10):3196-205. doi: 10.1021/ar5002507. Epub 2014 Sep 29. Acc Chem Res. 2014. PMID: 25262769 Review.
-
Mass Spectrometric Identification of [4Fe-4S](NO)x Intermediates of Nitric Oxide Sensing by Regulatory Iron-Sulfur Cluster Proteins.Chemistry. 2019 Mar 7;25(14):3675-3684. doi: 10.1002/chem.201806113. Epub 2019 Feb 7. Chemistry. 2019. PMID: 30600851
-
Mycobacterium tuberculosis WhiB3: a novel iron-sulfur cluster protein that regulates redox homeostasis and virulence.Antioxid Redox Signal. 2012 Apr 1;16(7):687-97. doi: 10.1089/ars.2011.4341. Antioxid Redox Signal. 2012. PMID: 22010944 Free PMC article. Review.
-
The function and properties of the iron-sulfur center in spinach ferredoxin: thioredoxin reductase: a new biological role for iron-sulfur clusters.Biochemistry. 1996 Sep 3;35(35):11425-34. doi: 10.1021/bi961007p. Biochemistry. 1996. PMID: 8784198
Cited by
-
Mycobacterium tuberculosis whiB3 and Lipid Metabolism Genes Are Regulated by Host Induced Oxidative Stress.Microorganisms. 2022 Sep 11;10(9):1821. doi: 10.3390/microorganisms10091821. Microorganisms. 2022. PMID: 36144423 Free PMC article.
-
Bacterial iron-sulfur regulatory proteins as biological sensor-switches.Antioxid Redox Signal. 2012 Nov 1;17(9):1215-31. doi: 10.1089/ars.2012.4511. Epub 2012 Mar 6. Antioxid Redox Signal. 2012. PMID: 22239203 Free PMC article. Review.
-
Clinically encountered growth phenotypes of tuberculosis-causing bacilli and their in vitro study: A review.Front Cell Infect Microbiol. 2022 Nov 10;12:1029111. doi: 10.3389/fcimb.2022.1029111. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439231 Free PMC article. Review.
-
Insights into redox sensing metalloproteins in Mycobacterium tuberculosis.J Inorg Biochem. 2014 Apr;133:118-26. doi: 10.1016/j.jinorgbio.2013.11.003. Epub 2013 Nov 15. J Inorg Biochem. 2014. PMID: 24314844 Free PMC article. Review.
-
Activation of Cryptic Antibiotic Biosynthetic Gene Clusters Guided by RNA-seq Data from Both Streptomyces ansochromogenes and ΔwblA.Antibiotics (Basel). 2021 Sep 10;10(9):1097. doi: 10.3390/antibiotics10091097. Antibiotics (Basel). 2021. PMID: 34572679 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

