Enhanced phosphorylation of transcription factor sp1 in response to herpes simplex virus type 1 infection is dependent on the ataxia telangiectasia-mutated protein
- PMID: 17609267
- PMCID: PMC2045397
- DOI: 10.1128/JVI.00568-07
Enhanced phosphorylation of transcription factor sp1 in response to herpes simplex virus type 1 infection is dependent on the ataxia telangiectasia-mutated protein
Abstract
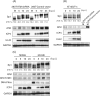
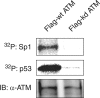
The ataxia telangiectasia-mutated (ATM) protein, a member of the related phosphatidylinositol 3-like kinase family encoded by a gene responsible for the human genetic disorder ataxia telangiectasia, regulates cellular responses to DNA damage and viral infection. It has been previously reported that herpes simplex virus type 1 (HSV-1) infection induces activation of protein kinase activity of ATM and hyperphosphorylation of transcription factor, Sp1. We show that ATM is intimately involved in Sp1 hyperphosphorylation during HSV-1 infection rather than individual HSV-1-encoded protein kinases. In ATM-deficient cells or cells silenced for ATM expression by short hairpin RNA targeting, hyperphosphorylation of Sp1 was prevented even as HSV-1 infection progressed. Mutational analysis of putative ATM phosphorylation sites on Sp1 and immunoblot analysis with phosphopeptide-specific Sp1 antibodies clarified that at least Ser-56 and Ser-101 residues on Sp1 became phosphorylated upon HSV-1 infection. Serine-to-alanine mutations at both sites on Sp1 considerably abolished hyperphosphorylation of Sp1 upon infection. Although ATM phosphorylated Ser-101 but not Ser-56 on Sp1 in vitro, phosphorylation of Sp1 at both sites was not detected at all upon infection in ATM-deficient cells, suggesting that cellular kinase(s) activated by ATM could be involved in phosphorylation at Ser-56. Upon viral infection, Sp1-dependent transcription in ATM expression-silenced cells was almost the same as that in ATM-intact cells, suggesting that ATM-dependent phosphorylation of Sp1 might hardly affect its transcriptional activity during the HSV-1 infection. ATM-dependent Sp1 phosphorylation appears to be a global response to various DNA damage stress including viral DNA replication.
Figures







Similar articles
-
Phosphorylation of Sp1 in response to DNA damage by ataxia telangiectasia-mutated kinase.Mol Cancer Res. 2007 Dec;5(12):1319-30. doi: 10.1158/1541-7786.MCR-07-0374. Mol Cancer Res. 2007. PMID: 18171990
-
Human cytomegalovirus disrupts both ataxia telangiectasia mutated protein (ATM)- and ATM-Rad3-related kinase-mediated DNA damage responses during lytic infection.J Virol. 2007 Feb;81(4):1934-50. doi: 10.1128/JVI.01670-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151099 Free PMC article.
-
Identification of phosphorylation sites on transcription factor Sp1 in response to DNA damage and its accumulation at damaged sites.Cell Signal. 2008 Oct;20(10):1795-803. doi: 10.1016/j.cellsig.2008.06.007. Epub 2008 Jun 19. Cell Signal. 2008. PMID: 18619531
-
The ATM-dependent DNA damage signaling pathway.Cold Spring Harb Symp Quant Biol. 2005;70:99-109. doi: 10.1101/sqb.2005.70.002. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869743 Review.
-
Molecular basis of ataxia telangiectasia and related diseases.Acta Pharmacol Sin. 2005 Aug;26(8):897-907. doi: 10.1111/j.1745-7254.2005.00165.x. Acta Pharmacol Sin. 2005. PMID: 16038621 Review.
Cited by
-
Post-translational control of sp-family transcription factors.Curr Genomics. 2008;9(5):301-11. doi: 10.2174/138920208785133244. Curr Genomics. 2008. PMID: 19471608 Free PMC article.
-
Measles Virus Infection Inactivates Cellular Protein Phosphatase 5 with Consequent Suppression of Sp1 and c-Myc Activities.J Virol. 2015 Oct;89(19):9709-18. doi: 10.1128/JVI.00825-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157124 Free PMC article.
-
Phosphorylation of transcriptional regulators in the retinoblastoma protein pathway by UL97, the viral cyclin-dependent kinase encoded by human cytomegalovirus.Virology. 2017 Dec;512:95-103. doi: 10.1016/j.virol.2017.09.009. Virology. 2017. PMID: 28946006 Free PMC article.
-
Epstein-Barr Virus Hijacks DNA Damage Response Transducers to Orchestrate Its Life Cycle.Viruses. 2017 Nov 16;9(11):341. doi: 10.3390/v9110341. Viruses. 2017. PMID: 29144413 Free PMC article. Review.
-
Role of ATM in the formation of the replication compartment during lytic replication of Epstein-Barr virus in nasopharyngeal epithelial cells.J Virol. 2015 Jan;89(1):652-68. doi: 10.1128/JVI.01437-14. Epub 2014 Oct 29. J Virol. 2015. PMID: 25355892 Free PMC article.
References
-
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
-
- Armstrong, S. A., D. A. Barry, R. W. Leggett, and C. R. Mueller. 1997. Casein kinase II-mediated phosphorylation of the C terminus of Sp1 decreases its DNA binding activity. J. Biol. Chem. 272:13489-13495. - PubMed
-
- Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. - PubMed
-
- Bouwman, P., and S. Philipsen. 2002. Regulation of the activity of Sp1-related transcription factors. Mol. Cell Endocrinol. 195:27-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

