Effects of intranasal administration of a leptin-secreting Lactococcus lactis recombinant on food intake, body weight, and immune response of mice
- PMID: 17601816
- PMCID: PMC1950963
- DOI: 10.1128/AEM.00295-07
Effects of intranasal administration of a leptin-secreting Lactococcus lactis recombinant on food intake, body weight, and immune response of mice
Abstract
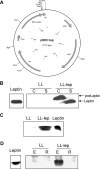
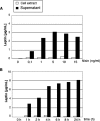
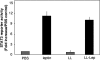
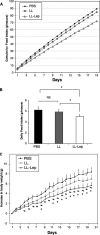
Leptin is an adipocyte-derived pleiotropic hormone that modulates a large number of physiological functions, including control of body weight and regulation of the immune system. In this work, we show that a recombinant strain of the food-grade lactic acid bacterium Lactococcus lactis (LL-lep) can produce and efficiently secrete human leptin. The secreted leptin is a fully biologically active hormone, as demonstrated by its capacity to stimulate a STAT3 reporter gene in HEK293 cells transfected with the Ob-Rb leptin receptor. The immunomodulatory activity of leptin-secreting L. lactis was evaluated in vivo by coexpression with the human papillomavirus type 16 E7 protein. In C57BL/6 mice immunized intranasally with a recombinant L. lactis strain coproducing leptin and E7 antigen, the adaptive immune response was significantly higher than in mice immunized with recombinant L. lactis producing only E7 antigen, demonstrating adjuvanticity of leptin. We then analyzed the effects of intranasally administered LL-lep in obese ob/ob mice. We observed that daily administration of LL-lep to these mice significantly reduced body weight gain and food intake. These results demonstrate that leptin can be produced and secreted in an active form by L. lactis and that leptin-producing L. lactis regulates in vivo antigen-specific immune responses, as well as body weight and food consumption.
Figures

Similar articles
-
Mucosal co-immunization of mice with recombinant lactococci secreting VapA antigen and leptin elicits a protective immune response against Rhodococcus equi infection.Vaccine. 2011 Dec 9;30(1):95-102. doi: 10.1016/j.vaccine.2011.10.026. Epub 2011 Oct 20. Vaccine. 2011. PMID: 22019740
-
Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria.Vaccine. 2007 Sep 4;25(36):6581-8. doi: 10.1016/j.vaccine.2007.06.062. Epub 2007 Jul 23. Vaccine. 2007. PMID: 17675182
-
Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production.Infect Immun. 2003 Apr;71(4):1887-96. doi: 10.1128/IAI.71.4.1887-1896.2003. Infect Immun. 2003. PMID: 12654805 Free PMC article.
-
Current prophylactic and therapeutic uses of a recombinant Lactococcus lactis strain secreting biologically active interleukin-12.J Mol Microbiol Biotechnol. 2008;14(1-3):80-9. doi: 10.1159/000106086. J Mol Microbiol Biotechnol. 2008. PMID: 17957114 Review.
-
Heterologous protein production and delivery systems for Lactococcus lactis.Genet Mol Res. 2003 Mar 31;2(1):102-11. Genet Mol Res. 2003. PMID: 12917806 Review.
Cited by
-
Induction of Immunogenic Response in BALB/c Mice by Live and Killed Form of Recombinant Lactococcus lactis Displaying EG95 of Echinococcus granulosus.Iran Biomed J. 2021 Jul 1;25(4):284-96. doi: 10.52547/ibj.25.4.284. Iran Biomed J. 2021. PMID: 34217159 Free PMC article.
-
Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines.Microb Cell Fact. 2011 Aug 30;10 Suppl 1(Suppl 1):S4. doi: 10.1186/1475-2859-10-S1-S4. Epub 2011 Aug 30. Microb Cell Fact. 2011. PMID: 21995317 Free PMC article. Review.
-
Recombinant Lactococcus lactis can make the difference in antigen-specific immune tolerance induction, the Type 1 Diabetes case.Microb Cell Fact. 2014 Aug 29;13 Suppl 1(Suppl 1):S11. doi: 10.1186/1475-2859-13-S1-S11. Epub 2014 Aug 29. Microb Cell Fact. 2014. PMID: 25185797 Free PMC article. Review.
-
[Use of lactic acid bacteria as mucosal vaccines].Rev Francoph Lab. 2009 Dec;2009(417):79-89. doi: 10.1016/S1773-035X(09)70312-0. Epub 2009 Dec 11. Rev Francoph Lab. 2009. PMID: 32518601 Free PMC article. French.
-
Exploiting Prophage-Mediated Lysis for Biotherapeutic Release by Lactobacillus reuteri.Appl Environ Microbiol. 2019 May 2;85(10):e02335-18. doi: 10.1128/AEM.02335-18. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30683744 Free PMC article.
References
-
- Ahima, R. S., and S. Y. Osei. 2004. Leptin signaling. Physiol. Behav. 81:223-241. - PubMed
-
- Amer, A., and T. J. Maher. 2005. Nasal administration of the calcium channel blocker diltiazem decreases food intake and attenuates weight gain in rats. Pharmacol. Biochem. Behav. 82:379-387. - PubMed
-
- Bermudez-Humaran, L. G., N. G. Cortes-Perez, Y. Le Loir, A. Gruss, C. Rodriguez-Padilla, O. Saucedo-Cardenas, P. Langella, and R. Montes de Oca-Luna. 2003. Fusion to a carrier protein and a synthetic propeptide enhances E7 HPV-16 production and secretion in Lactococcus lactis. Biotechnol. Prog. 19:1101-1104. - PubMed
-
- Bermudez-Humaran, L. G., N. G. Cortes-Perez, F. Lefevre, V. Guimaraes, S. Rabot, J. M. Alcocer-Gonzalez, J. J. Gratadoux, C. Rodriguez-Padilla, R. S. Tamez-Guerra, G. Corthier, A. Gruss, and P. Langella. 2005. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 175:7297-7302. - PubMed
-
- Bermudez-Humaran, L. G., P. Langella, J. Commissaire, S. Gilbert, Y. Le Loir, R. L'Haridon, and G. Corthier. 2003. Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis. FEMS Microbiol. Lett. 224:307-313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

