Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer's disease-linked mutant presenilin 1
- PMID: 17596450
- PMCID: PMC2801050
- DOI: 10.1523/JNEUROSCI.4272-06.2007
Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer's disease-linked mutant presenilin 1
Abstract
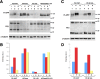
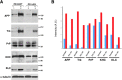
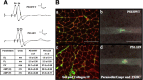
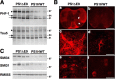
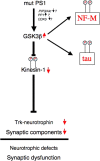
Presenilins (PS) play a central role in gamma-secretase-mediated processing of beta-amyloid precursor protein (APP) and numerous type I transmembrane proteins. Expression of mutant PS1 variants causes familial forms of Alzheimer's disease (FAD). In cultured mammalian cells that express FAD-linked PS1 variants, the intracellular trafficking of several type 1 membrane proteins is altered. We now report that the anterograde fast axonal transport (FAT) of APP and Trk receptors is impaired in the sciatic nerves of transgenic mice expressing two independent FAD-linked PS1 variants. Furthermore, FAD-linked PS1 mice exhibit a significant increase in phosphorylation of the cytoskeletal proteins tau and neurofilaments in the spinal cord. Reductions in FAT and phosphorylation abnormalities correlated with motor neuron functional deficits. Together, our data suggests that defects in anterograde FAT may underlie FAD-linked PS1-mediated neurodegeneration through a mechanism involving impairments in neurotrophin signaling and synaptic dysfunction.
Figures

Similar articles
-
Presenilin transgenic mice as models of Alzheimer's disease.Brain Struct Funct. 2010 Mar;214(2-3):127-43. doi: 10.1007/s00429-009-0227-3. Epub 2009 Nov 18. Brain Struct Funct. 2010. PMID: 19921519 Free PMC article. Review.
-
Alzheimer's presenilin 1 modulates sorting of APP and its carboxyl-terminal fragments in cerebral neurons in vivo.J Neurochem. 2007 Aug;102(3):619-26. doi: 10.1111/j.1471-4159.2007.04587.x. J Neurochem. 2007. PMID: 17630980
-
Decreased Abeta secretion by cells expressing familial Alzheimer's disease-linked mutant presenilin 1.Neurosci Res. 2007 Mar;57(3):446-53. doi: 10.1016/j.neures.2006.12.005. Epub 2007 Jan 8. Neurosci Res. 2007. PMID: 17210196
-
Deficits in Enrichment-Dependent Neurogenesis and Enhanced Anxiety Behaviors Mediated by Expression of Alzheimer's Disease-Linked Ps1 Variants Are Rescued by Microglial Depletion.J Neurosci. 2019 Aug 21;39(34):6766-6780. doi: 10.1523/JNEUROSCI.0884-19.2019. Epub 2019 Jun 19. J Neurosci. 2019. PMID: 31217332 Free PMC article.
-
Transgenic mouse models of Alzheimer's disease: phenotype and mechanisms of pathogenesis.Biochem Soc Symp. 2001;(67):195-202. doi: 10.1042/bss0670195. Biochem Soc Symp. 2001. PMID: 11447835 Review.
Cited by
-
Transient focal cerebral ischemia/reperfusion induces early and chronic axonal changes in rats: its importance for the risk of Alzheimer's disease.PLoS One. 2012;7(3):e33722. doi: 10.1371/journal.pone.0033722. Epub 2012 Mar 23. PLoS One. 2012. PMID: 22457786 Free PMC article.
-
Axonal transport defects in neurodegenerative diseases.J Neurosci. 2009 Oct 14;29(41):12776-86. doi: 10.1523/JNEUROSCI.3463-09.2009. J Neurosci. 2009. PMID: 19828789 Free PMC article. Review.
-
Presenilin transgenic mice as models of Alzheimer's disease.Brain Struct Funct. 2010 Mar;214(2-3):127-43. doi: 10.1007/s00429-009-0227-3. Epub 2009 Nov 18. Brain Struct Funct. 2010. PMID: 19921519 Free PMC article. Review.
-
Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration.J Neurosci. 2009 Jan 7;29(1):118-30. doi: 10.1523/JNEUROSCI.3985-08.2009. J Neurosci. 2009. PMID: 19129390 Free PMC article.
-
Alzheimer's Disease: From Mitochondrial Perturbations to Mitochondrial Medicine.Brain Pathol. 2016 Sep;26(5):632-47. doi: 10.1111/bpa.12402. Brain Pathol. 2016. PMID: 27327899 Free PMC article. Review.
References
-
- Annaert WG, Esselens C, Baert V, Boeve C, Snellings G, Cupers P, Craessaerts K, De Strooper B. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron. 2001;32:579–589. - PubMed
-
- Assini A, Terreni L, Borghi R, Giliberto L, Piccini A, Loqui D, Fogliarino S, Forloni G, Tabaton M. Pure spastic paraparesis associated with a novel presenilin 1 R278K mutation. Neurology. 2003;60:150. - PubMed
-
- Bajaj NP, Miller CC. Phosphorylation of neurofilament heavy-chain side-arm fragments by cyclin-dependent kinase-5 and glycogen synthase kinase-3alpha in transfected cells. J Neurochem. 1997;69:737–743. - PubMed
-
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS043408/NS/NINDS NIH HHS/United States
- R56 NS023868/NS/NINDS NIH HHS/United States
- R01 AG021494/AG/NIA NIH HHS/United States
- AG021494/AG/NIA NIH HHS/United States
- NS41170/NS/NINDS NIH HHS/United States
- R01 NS023320/NS/NINDS NIH HHS/United States
- NS43408/NS/NINDS NIH HHS/United States
- R01 NS041170/NS/NINDS NIH HHS/United States
- NS23868/NS/NINDS NIH HHS/United States
- R01 NS023868/NS/NINDS NIH HHS/United States
- R01 AG033570/AG/NIA NIH HHS/United States
- NS23320/NS/NINDS NIH HHS/United States
- R01 NS023868-20/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
