Targeted dendrotomy reveals active and passive contributions of the dendritic tree to synaptic integration and neuronal output
- PMID: 17592119
- PMCID: PMC2040918
- DOI: 10.1073/pnas.0701586104
Targeted dendrotomy reveals active and passive contributions of the dendritic tree to synaptic integration and neuronal output
Abstract
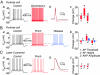
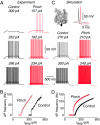
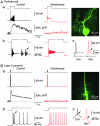
Neurons typically function as transduction devices, converting patterns of synaptic inputs, received on the dendrites, into trains of output action potentials in the axon. This transduction process is surprisingly complex and has been proposed to involve a two-way dialogue between axosomatic and dendritic compartments that can generate mutually interacting regenerative responses. To manipulate this process, we have developed a new approach for rapid and reversible occlusion or amputation of the primary dendrites of individual neurons in brain slices. By applying these techniques to cerebellar Purkinje and layer 5 cortical pyramidal neurons, we show directly that both the active and passive properties of dendrites differentially affect firing in the axon depending on the strength of stimulation. For weak excitation, dendrites act as a passive electrical load, raising spike threshold and dampening axonal excitability. For strong excitation, dendrites contribute regenerative inward currents, which trigger burst firing and enhance neuronal excitability. These findings provide direct support for the idea that dendritic morphology and conductances act in concert to regulate the excitability of the neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Synaptic integration in dendritic trees.J Neurobiol. 2005 Jul;64(1):75-90. doi: 10.1002/neu.20144. J Neurobiol. 2005. PMID: 15884003 Review.
-
Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle.Science. 2009 Aug 7;325(5941):756-60. doi: 10.1126/science.1171958. Science. 2009. PMID: 19661433
-
Reliable control of spike rate and spike timing by rapid input transients in cerebellar stellate cells.Neuroscience. 2004;124(2):305-17. doi: 10.1016/j.neuroscience.2003.11.015. Neuroscience. 2004. PMID: 14980381
-
A novel role for MNTB neuron dendrites in regulating action potential amplitude and cell excitability during repetitive firing.Eur J Neurosci. 2008 Jun;27(12):3095-108. doi: 10.1111/j.1460-9568.2008.06297.x. Eur J Neurosci. 2008. PMID: 18598256
-
Influence of dendritic conductances on the input-output properties of neurons.Annu Rev Neurosci. 2001;24:653-75. doi: 10.1146/annurev.neuro.24.1.653. Annu Rev Neurosci. 2001. PMID: 11520915 Review.
Cited by
-
Disruption of metabotropic glutamate receptor signalling is a major defect at cerebellar parallel fibre-Purkinje cell synapses in staggerer mutant mice.J Physiol. 2011 Jul 1;589(Pt 13):3191-209. doi: 10.1113/jphysiol.2011.207563. Epub 2011 May 9. J Physiol. 2011. PMID: 21558162 Free PMC article.
-
Divide et impera: optimizing compartmental models of neurons step by step.J Physiol. 2009 Apr 1;587(Pt 7):1369-70. doi: 10.1113/jphysiol.2009.170944. J Physiol. 2009. PMID: 19336603 Free PMC article. No abstract available.
-
Physiological temperature during brain slicing enhances the quality of acute slice preparations.Front Cell Neurosci. 2013 Apr 23;7:48. doi: 10.3389/fncel.2013.00048. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23630465 Free PMC article.
-
Electrophysiological classes of layer 2/3 pyramidal cells in monkey prefrontal cortex.J Neurophysiol. 2012 Jul;108(2):595-609. doi: 10.1152/jn.00859.2011. Epub 2012 Apr 11. J Neurophysiol. 2012. PMID: 22496534 Free PMC article.
-
A biologically inspired repair mechanism for neuronal reconstructions with a focus on human dendrites.PLoS Comput Biol. 2024 Feb 23;20(2):e1011267. doi: 10.1371/journal.pcbi.1011267. eCollection 2024 Feb. PLoS Comput Biol. 2024. PMID: 38394339 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

