Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity
- PMID: 17591961
- PMCID: PMC1941581
- DOI: 10.2353/ajpath.2007.061116
Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity
Abstract
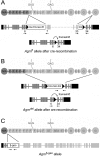
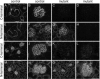
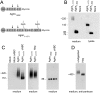
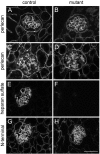
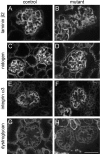
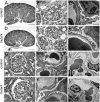
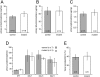
Glomerular charge selectivity has been attributed to anionic heparan sulfate proteoglycans (HSPGs) in the glomerular basement membrane (GBM). Agrin is the predominant GBM-HSPG, but evidence that it contributes to the charge barrier is lacking, because newborn agrin-deficient mice die from neuromuscular defects. To study agrin in adult kidney, a new conditional allele was used to generate podocyte-specific knockouts. Mutants were viable and displayed no renal histopathology up to 9 months of age. Perlecan, a HSPG normally confined to the mesangium in mature glomeruli, did not appear in the mutant GBM, which lacked heparan sulfate. Moreover, GBM agrin was found to be derived primarily from podocytes. Polyethyleneimine labeling of fetal kidneys revealed anionic sites along both laminae rarae of the GBM that became most prominent along the subepithelial aspect at maturity; labeling was greatly reduced along the subepithelial aspect in agrin-deficient and conditional knockout mice. Despite this severe charge disruption, the glomerular filtration barrier was not compromised, even when challenged with bovine serum albumin overload. We conclude that agrin is not required for establishment or maintenance of GBM architecture. Although agrin contributes significantly to the anionic charge to the GBM, both it and its charge are not needed for glomerular permselectivity. This calls into question whether charge selectivity is a feature of the GBM.
Figures

Comment in
-
Contribution of proteoglycans towards the integrated functions of renal glomerular capillaries: a historical perspective.Am J Pathol. 2007 Jul;171(1):9-13. doi: 10.2353/ajpath.2007.070356. Am J Pathol. 2007. PMID: 17591948 Free PMC article. Review. No abstract available.
Similar articles
-
Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane.Nephrol Dial Transplant. 2009 Jul;24(7):2044-51. doi: 10.1093/ndt/gfn758. Epub 2009 Jan 14. Nephrol Dial Transplant. 2009. PMID: 19144998 Free PMC article.
-
Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane.J Histochem Cytochem. 1998 Jan;46(1):19-27. doi: 10.1177/002215549804600104. J Histochem Cytochem. 1998. PMID: 9405491
-
The reduction of heparan sulphate in the glomerular basement membrane does not augment urinary albumin excretion.Nephrol Dial Transplant. 2018 Jan 1;33(1):26-33. doi: 10.1093/ndt/gfx218. Nephrol Dial Transplant. 2018. PMID: 28992095
-
Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane.Nephrol Dial Transplant. 1999 Sep;14(9):2119-29. doi: 10.1093/ndt/14.9.2119. Nephrol Dial Transplant. 1999. PMID: 10489220 Review.
-
Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation.Matrix Biol. 2017 Jan;57-58:299-310. doi: 10.1016/j.matbio.2016.09.002. Epub 2016 Sep 6. Matrix Biol. 2017. PMID: 27609404 Free PMC article. Review.
Cited by
-
New role for Agrin in T cells and its potential importance in immune system regulation.Arthritis Res Ther. 2010;12(2):205. doi: 10.1186/ar2957. Epub 2010 Apr 12. Arthritis Res Ther. 2010. PMID: 20398335 Free PMC article. Review.
-
Heparanase induces a differential loss of heparan sulphate domains in overt diabetic nephropathy.Diabetologia. 2008 Feb;51(2):372-82. doi: 10.1007/s00125-007-0879-6. Epub 2007 Dec 6. Diabetologia. 2008. PMID: 18058084
-
LMX1B is essential for the maintenance of differentiated podocytes in adult kidneys.J Am Soc Nephrol. 2013 Nov;24(11):1830-48. doi: 10.1681/ASN.2012080788. Epub 2013 Aug 29. J Am Soc Nephrol. 2013. PMID: 23990680 Free PMC article.
-
A deletion in the N-terminal polymerizing domain of laminin β2 is a new mouse model of chronic nephrotic syndrome.Kidney Int. 2020 Jul;98(1):133-146. doi: 10.1016/j.kint.2020.01.033. Epub 2020 Feb 20. Kidney Int. 2020. PMID: 32456966 Free PMC article.
-
Single-cell transcriptome atlas in C57BL/6 mice encodes morphological phenotypes in the aging kidneys.BMC Nephrol. 2024 Apr 19;25(1):137. doi: 10.1186/s12882-024-03514-0. BMC Nephrol. 2024. PMID: 38641839 Free PMC article.
References
-
- Chang RL, Deen WM, Robertson CR, Brenner BM. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975;8:212–218. - PubMed
-
- Rennke HG, Patel Y, Venkatachalam MA. Glomerular filtration of proteins: clearance of anionic, neutral and cationic horseradish peroxidase in the rat. Kidney Int. 1978;13:278–288. - PubMed
-
- Comper WD, Glascow EF. Charge selectivity in kidney ultrafiltration. Kidney Int. 1995;47:1242–1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

