Macrophages transmit human immunodeficiency virus type 1 products to CD4-negative cells: involvement of matrix metalloproteinase 9
- PMID: 17581988
- PMCID: PMC1951421
- DOI: 10.1128/JVI.00675-07
Macrophages transmit human immunodeficiency virus type 1 products to CD4-negative cells: involvement of matrix metalloproteinase 9
Abstract
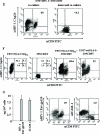
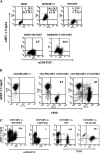
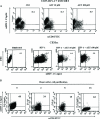
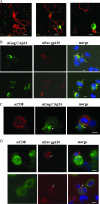
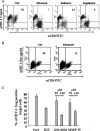
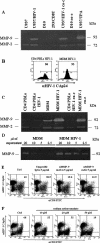
It was previously reported that human immunodeficiency virus type 1 (HIV-1) spreads in CD4 lymphocytes through cell-to-cell transmission. Here we report that HIV-1-infected macrophages, but not lymphocytes, transmit HIV-1 products to CD4-negative cells of either epithelial, neuronal, or endothelial origin in the absence of overt HIV-1 infection. This phenomenon was detectable as early as 1 h after the start of cocultivation and depended on cell-to-cell contact but not on the release of viral particles from donor cells. Transfer of HIV-1 products occurred upon their polarization and colocalization within zones of cell-to-cell contact similar to virological synapses. Neither HIV-1 Env nor Nef expression was required but, interestingly, we found that an HIV-1-dependent increase in matrix metalloproteinase 9 production from donor cells significantly contributed to the cell-to-cell transmission of the viral products. The macrophage-driven transfer of HIV-1 products to diverse CD4-negative cell types may have a significant role in AIDS pathogenesis.
Figures

Similar articles
-
HLA-A2 down-regulation on primary human macrophages infected with an M-tropic EGFP-tagged HIV-1 reporter virus.J Leukoc Biol. 2005 Sep;78(3):675-85. doi: 10.1189/jlb.0505237. Epub 2005 Jul 6. J Leukoc Biol. 2005. PMID: 16000390
-
HIV-1-infected macrophages induce astrogliosis by SDF-1alpha and matrix metalloproteinases.Biochem Biophys Res Commun. 2005 Nov 4;336(4):1214-20. doi: 10.1016/j.bbrc.2005.08.251. Biochem Biophys Res Commun. 2005. PMID: 16169519
-
HIV-1 Nef mediates post-translational down-regulation and redistribution of the mannose receptor.J Leukoc Biol. 2005 Apr;77(4):522-34. doi: 10.1189/jlb.0804454. Epub 2005 Jan 6. J Leukoc Biol. 2005. PMID: 15637102
-
Human Polycomb group EED protein negatively affects HIV-1 assembly and release.Retrovirology. 2007 Jun 4;4:37. doi: 10.1186/1742-4690-4-37. Retrovirology. 2007. PMID: 17547741 Free PMC article.
-
Human immunodeficiency virus type 1 infection of human uterine epithelial cells: viral shedding and cell contact-mediated infectivity.J Infect Dis. 2003 May 15;187(10):1522-33. doi: 10.1086/374782. Epub 2003 Apr 23. J Infect Dis. 2003. PMID: 12721932
Cited by
-
Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study.J Neurovirol. 2018 Feb;24(1):41-51. doi: 10.1007/s13365-017-0590-4. Epub 2017 Oct 23. J Neurovirol. 2018. PMID: 29063513 Free PMC article.
-
Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism.J Virol. 2014 Oct;88(19):11529-39. doi: 10.1128/JVI.01712-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056899 Free PMC article.
-
HIV-1 Nef in macrophage-mediated disease pathogenesis.Int Rev Immunol. 2012 Dec;31(6):432-50. doi: 10.3109/08830185.2012.737073. Int Rev Immunol. 2012. PMID: 23215766 Free PMC article. Review.
-
Tubular cell HIV-entry through apoptosed CD4 T cells: a novel pathway.Virology. 2012 Dec 5;434(1):68-77. doi: 10.1016/j.virol.2012.09.009. Epub 2012 Oct 3. Virology. 2012. PMID: 23040891 Free PMC article.
-
HIV-associated neuropathogenesis: a systems biology perspective for modeling and therapy.Biosystems. 2014 May;119:53-61. doi: 10.1016/j.biosystems.2014.04.002. Epub 2014 Apr 13. Biosystems. 2014. PMID: 24732754 Free PMC article. Review.
References
-
- Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257-6265. - PubMed
-
- Ambrosini, E., N. Slepko, B. Kohleisen, E. Shumay, V. Erfle, F. Aloisi, and G. Levi. 1999. HIV-1 Nef alters the expression of betaII and epsilon isoforms of protein kinase C and the activation of the long terminal repeat promoter in human astrocytoma cells. Glia 27:143-151. - PubMed
-
- Andre, P., M. Groettrup, P. Klenerman, R. de Guili, B. L. Booth, Jr., V. Cerundolo, M. Bonneville, F. Jotereau, R. M. Zinkernagel, and V. Lotteau. 1998. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc. Natl. Acad. Sci. USA 95:13120-13124. - PMC - PubMed
-
- Blanco, J., B. Bosch, M. T. Fernandez-Figueras, J. Barretina, B. Clotet, and J. A. Este. 2004. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J. Biol. Chem. 279:51305-51314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

