Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR
- PMID: 17581630
- PMCID: PMC1933400
- DOI: 10.1038/sj.emboj.7601769
Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR
Abstract
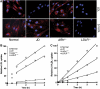
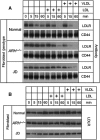
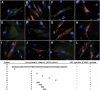
The low-density lipoprotein (LDL) receptor (LDLR) binds to and internalizes lipoproteins that contain apolipoproteinB100 (apoB100) or apolipoproteinE (apoE). Internalization of the apoB100 lipoprotein ligand, LDL, requires the FDNPVY(807) sequence on the LDLR cytoplasmic domain, which binds to the endocytic machinery of coated pits. We show here that inactivation of the FDNPVY(807) sequence by mutation of Y807 to cysteine prevented the uptake of LDL; however, this mutation did not prevent LDLR-dependent uptake of the apoE lipoprotein ligand, beta-VLDL. Comparison of the surface localization of the LDLR-Y807C using LDLR-immunogold, LDL-gold and beta-VLDL-gold probes revealed enrichment of LDLR-Y807C-bound beta-VLDL in coated pits, suggesting that beta-VLDL binding promoted the internalization of the LDLR-Y807C. Consistent with this possibility, treatment with monensin, which traps internalized LDLR in endosomes, resulted in the loss of surface LDLR-Y807C only when beta-VLDL was present. Reconstitution experiments in which LDLR variants were introduced into LDLR-deficient cells showed that the HIC(818) sequence is involved in beta-VLDL uptake by the LDLR-Y807C. Together, these experiments demonstrate that the LDLR has a very low-density lipoprotein (VLDL)-induced, FDNPVY-independent internalization mechanism.
Figures



Similar articles
-
Morphological characterization of beta-VLDL and acetylated-LDL binding and internalization by cultured pigeon monocytes.Exp Mol Pathol. 1989 Dec;51(3):243-63. doi: 10.1016/0014-4800(89)90023-3. Exp Mol Pathol. 1989. PMID: 2513225
-
Absence of hyperlipidemia in LDL receptor-deficient mice having apolipoprotein B100 without the putative receptor-binding sequences.Arterioscler Thromb Vasc Biol. 2008 Oct;28(10):1745-52. doi: 10.1161/ATVBAHA.108.169680. Epub 2008 Jul 10. Arterioscler Thromb Vasc Biol. 2008. PMID: 18617647 Free PMC article.
-
The modular adaptor protein autosomal recessive hypercholesterolemia (ARH) promotes low density lipoprotein receptor clustering into clathrin-coated pits.J Biol Chem. 2005 Dec 9;280(49):40996-1004. doi: 10.1074/jbc.M509394200. Epub 2005 Sep 22. J Biol Chem. 2005. PMID: 16179341
-
Compensated endocytosis of LDL by hamster cells co-expressing the two distinct mutant LDL receptors defective in endocytosis and ligand binding.J Lipid Res. 1999 May;40(5):814-23. J Lipid Res. 1999. PMID: 10224150
-
Structure and physiologic function of the low-density lipoprotein receptor.Annu Rev Biochem. 2005;74:535-62. doi: 10.1146/annurev.biochem.74.082803.133354. Annu Rev Biochem. 2005. PMID: 15952897 Review.
Cited by
-
Quantitative fluorescence imaging reveals point of release for lipoproteins during LDLR-dependent uptake.J Lipid Res. 2013 Mar;54(3):744-753. doi: 10.1194/jlr.M033548. Epub 2013 Jan 7. J Lipid Res. 2013. PMID: 23296879 Free PMC article.
-
S-nitrosylation of ARH is required for LDL uptake by the LDL receptor.J Lipid Res. 2013 Jun;54(6):1550-1559. doi: 10.1194/jlr.M033167. Epub 2013 Apr 7. J Lipid Res. 2013. PMID: 23564733 Free PMC article.
-
Mechanisms and genetic determinants regulating sterol absorption, circulating LDL levels, and sterol elimination: implications for classification and disease risk.J Lipid Res. 2011 Nov;52(11):1885-926. doi: 10.1194/jlr.R017855. Epub 2011 Aug 23. J Lipid Res. 2011. PMID: 21862702 Free PMC article. Review.
-
Low Expression of Sirtuin 1 in the Dairy Cows with Mild Fatty Liver Alters Hepatic Lipid Metabolism.Animals (Basel). 2020 Mar 27;10(4):560. doi: 10.3390/ani10040560. Animals (Basel). 2020. PMID: 32230804 Free PMC article.
-
The LXR-IDOL axis defines a clathrin-, caveolae-, and dynamin-independent endocytic route for LDLR internalization and lysosomal degradation.J Lipid Res. 2013 Aug;54(8):2174-2184. doi: 10.1194/jlr.M037713. Epub 2013 Jun 3. J Lipid Res. 2013. PMID: 23733886 Free PMC article.
References
-
- Anderson RG, Goldstein JL, Brown MS (1977) A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature 270: 695–699 - PubMed
-
- Arca M, Zuliani G, Wilund K, Campagna F, Fellin R, Bertolini S, Calandra S, Ricci G, Glorioso N, Maioli M, Pintus P, Carru C, Cossu F, Cohen J, Hobbs HH (2002) Autosomal recessive hypercholesterolaemia in Sardinia, Italy, and mutations in ARH: a clinical and molecular genetic analysis. Lancet 359: 841–847 - PubMed
-
- Basu SK, Goldstein JL, Anderson RG, Brown MS (1981) Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24: 493–502 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

