Post-infection immunodeficiency virus control by neutralizing antibodies
- PMID: 17579714
- PMCID: PMC1890307
- DOI: 10.1371/journal.pone.0000540
Post-infection immunodeficiency virus control by neutralizing antibodies
Abstract
Background: Unlike most acute viral infections controlled with the appearance of virus-specific neutralizing antibodies (NAbs), primary HIV infections are not met with such potent and early antibody responses. This brings into question if or how the presence of potent antibodies can contribute to primary HIV control, but protective efficacies of antiviral antibodies in primary HIV infections have remained elusive; and, it has been speculated that even NAb induction could have only a limited suppressive effect on primary HIV replication once infection is established. Here, in an attempt to answer this question, we examined the effect of passive NAb immunization post-infection on primary viral replication in a macaque AIDS model.
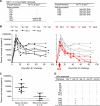
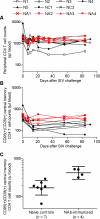
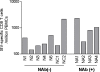
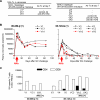
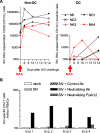
Methods and findings: The inoculums for passive immunization with simian immunodeficiency virus mac239 (SIVmac239)-specific neutralizing activity were prepared by purifying polyclonal immunoglobulin G from pooled plasma of six SIVmac239-infected rhesus macaques with NAb induction in the chronic phase. Passive immunization of rhesus macaques with the NAbs at day 7 after SIVmac239 challenge resulted in significant reduction of set-point plasma viral loads and preservation of central memory CD4 T lymphocyte counts, despite the limited detection period of the administered NAb responses. Peripheral lymph node dendritic cell (DC)-associated viral RNA loads showed a remarkable peak with the NAb administration, and DCs stimulated in vitro with NAb-preincubated SIV activated virus-specific CD4 T lymphocytes in an Fc-dependent manner, implying antibody-mediated virion uptake by DCs and enhanced T cell priming.
Conclusions: Our results present evidence indicating that potent antibody induction post-infection can result in primary immunodeficiency virus control and suggest direct and indirect contribution of its absence to initial control failure in HIV infections. Although difficulty in achieving requisite neutralizing titers for sterile HIV protection by prophylactic vaccination has been suggested, this study points out a possibility of non-sterile HIV control by prophylactic vaccine-induced, sub-sterile titers of NAbs post-infection, providing a rationale of vaccine-based NAb induction for primary HIV control.
Conflict of interest statement
Figures
Similar articles
-
Patterns of HIV/SIV Prevention and Control by Passive Antibody Immunization.Front Microbiol. 2016 Nov 2;7:1739. doi: 10.3389/fmicb.2016.01739. eCollection 2016. Front Microbiol. 2016. PMID: 27853456 Free PMC article. Review.
-
Biphasic CD8+ T-Cell Defense in Simian Immunodeficiency Virus Control by Acute-Phase Passive Neutralizing Antibody Immunization.J Virol. 2016 Jun 24;90(14):6276-6290. doi: 10.1128/JVI.00557-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122584 Free PMC article.
-
Limited impact of passive non-neutralizing antibody immunization in acute SIV infection on viremia control in rhesus macaques.PLoS One. 2013 Sep 9;8(9):e73453. doi: 10.1371/journal.pone.0073453. eCollection 2013. PLoS One. 2013. PMID: 24039947 Free PMC article.
-
Neutralizing antibodies in SIV control: co-impact with T cells.Vaccine. 2010 May 26;28 Suppl 2:B13-7. doi: 10.1016/j.vaccine.2009.09.080. Vaccine. 2010. PMID: 20510737
-
Fc receptor-mediated antiviral antibodies.Curr Opin HIV AIDS. 2009 Sep;4(5):388-93. doi: 10.1097/COH.0b013e32832f0a89. Curr Opin HIV AIDS. 2009. PMID: 20048702 Free PMC article. Review.
Cited by
-
Antibody-Dependent Cellular Cytotoxicity and NK Cell-Driven Immune Escape in HIV Infection: Implications for HIV Vaccine Development.Adv Virol. 2012;2012:637208. doi: 10.1155/2012/637208. Epub 2012 Apr 29. Adv Virol. 2012. PMID: 22611395 Free PMC article.
-
Patterns of HIV/SIV Prevention and Control by Passive Antibody Immunization.Front Microbiol. 2016 Nov 2;7:1739. doi: 10.3389/fmicb.2016.01739. eCollection 2016. Front Microbiol. 2016. PMID: 27853456 Free PMC article. Review.
-
Elite control of HIV infection: implications for vaccine design.Expert Opin Biol Ther. 2009 Jan;9(1):55-69. doi: 10.1517/14712590802571928. Expert Opin Biol Ther. 2009. PMID: 19063693 Free PMC article. Review.
-
Antibody-mediated prevention and treatment of HIV-1 infection.Retrovirology. 2018 Nov 16;15(1):73. doi: 10.1186/s12977-018-0455-9. Retrovirology. 2018. PMID: 30445968 Free PMC article. Review.
-
Control of simian immunodeficiency virus replication by vaccine-induced Gag- and Vif-specific CD8+ T cells.J Virol. 2014 Jan;88(1):425-33. doi: 10.1128/JVI.02634-13. Epub 2013 Oct 23. J Virol. 2014. PMID: 24155398 Free PMC article.
References
-
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

