Lung macrophages from bacille Calmette-Guérin-vaccinated guinea pigs suppress T cell proliferation but restrict intracellular growth of M. tuberculosis after recombinant guinea pig interferon-gamma activation
- PMID: 17565610
- PMCID: PMC1941958
- DOI: 10.1111/j.1365-2249.2007.03425.x
Lung macrophages from bacille Calmette-Guérin-vaccinated guinea pigs suppress T cell proliferation but restrict intracellular growth of M. tuberculosis after recombinant guinea pig interferon-gamma activation
Abstract
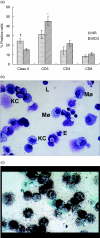
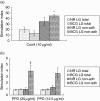
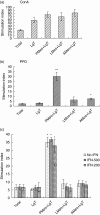
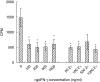
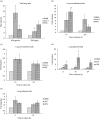
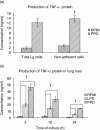
The guinea pig model of low-dose pulmonary tuberculosis has been used to study the pathogenesis of infection as well as the mechanisms of bacille Calmette-Guérin (BCG) vaccine-induced resistance. We investigated the function of lung cells from naive and BCG-vaccinated guinea pigs after enzymatic digestion of lung tissue with collagenase and DNase I. The total lung digest cells proliferated poorly to purified protein derivative (PPD) but comparatively better to ConA as assessed by [(3)H]-thymidine uptake. However, the non-adherent population obtained after plastic adherence of lung digests showed an enhanced response to concanavalin A (ConA) and PPD. Therefore, proliferation to ConA and PPD of nylon wool-purified T cells co-cultured with peritoneal (PMøs), alveolar (AMøs) or lung macrophages (LMøs) was assessed. Co-cultures of lung T cells and PMøs showed maximum proliferation to PPD, whereas proliferation was suppressed significantly by the addition of AMøs or LMøs. The response of T cells to ConA was unaffected in co-cultures. Incubation of co-cultures with recombinant guinea pig interferon-gamma (rgpIFN-gamma) did not reverse the suppression. In contrast, rgpIFN-gamma-treated plastic adherent LMøs that were non-specific esterase-positive were capable of reducing the intracellular growth of Mycobacterium tuberculosis. Similarly, total, non-adherent and adherent lung digest cells from BCG-vaccinated guinea pigs showed IFN-gamma and tumour necrosis factor (TNF)-alpha mRNA expression in response to ConA, lipopolysaccharide or PPD by reverse transcription-polymerase chain reaction followed by release of TNF protein but not IFN. These studies indicate that rgp-IFN-gamma-treated lung tissue macrophages from BCG-vaccinated guinea pigs are defective for inducing antigen-specific proliferation in T cells, but control the intracellular accumulation of virulent M. tuberculosis.
Figures
Similar articles
-
Expression of interferon-gamma and tumour necrosis factor-alpha messenger RNA does not correlate with protection in guinea pigs challenged with virulent Mycobacterium tuberculosis by the respiratory route.Immunology. 2009 Sep;128(1 Suppl):e296-305. doi: 10.1111/j.1365-2567.2008.02962.x. Epub 2008 Nov 7. Immunology. 2009. PMID: 19016908 Free PMC article.
-
The in vivo immunomodulatory effect of recombinant tumour necrosis factor-alpha in guinea pigs vaccinated with Mycobacterium bovis bacille Calmette-Guérin.Clin Exp Immunol. 2011 Jul;165(1):110-20. doi: 10.1111/j.1365-2249.2011.04406.x. Epub 2011 May 5. Clin Exp Immunol. 2011. PMID: 21545584 Free PMC article.
-
Recombinant guinea pig TNF-alpha enhances antigen-specific type 1 T lymphocyte activation in guinea pig splenocytes.Tuberculosis (Edinb). 2007 Mar;87(2):87-93. doi: 10.1016/j.tube.2005.12.001. Epub 2006 Aug 8. Tuberculosis (Edinb). 2007. PMID: 16899409
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Development of an asthma vaccine: research into BCG.Drugs. 2000 Jun;59(6):1217-21. doi: 10.2165/00003495-200059060-00002. Drugs. 2000. PMID: 10882158 Review.
Cited by
-
The Progress of Therapeutic Vaccination with Regard to Tuberculosis.Front Microbiol. 2016 Sep 28;7:1536. doi: 10.3389/fmicb.2016.01536. eCollection 2016. Front Microbiol. 2016. PMID: 27733848 Free PMC article. Review.
-
Expression of interferon-gamma and tumour necrosis factor-alpha messenger RNA does not correlate with protection in guinea pigs challenged with virulent Mycobacterium tuberculosis by the respiratory route.Immunology. 2009 Sep;128(1 Suppl):e296-305. doi: 10.1111/j.1365-2567.2008.02962.x. Epub 2008 Nov 7. Immunology. 2009. PMID: 19016908 Free PMC article.
-
Progressive Host-Directed Strategies to Potentiate BCG Vaccination Against Tuberculosis.Front Immunol. 2022 Jul 28;13:944183. doi: 10.3389/fimmu.2022.944183. eCollection 2022. Front Immunol. 2022. PMID: 35967410 Free PMC article. Review.
-
The in vivo immunomodulatory effect of recombinant tumour necrosis factor-alpha in guinea pigs vaccinated with Mycobacterium bovis bacille Calmette-Guérin.Clin Exp Immunol. 2011 Jul;165(1):110-20. doi: 10.1111/j.1365-2249.2011.04406.x. Epub 2011 May 5. Clin Exp Immunol. 2011. PMID: 21545584 Free PMC article.
-
Neutralization of TNFalpha alters inflammation in guinea pig tuberculous pleuritis.Microbes Infect. 2009 May-Jun;11(6-7):680-8. doi: 10.1016/j.micinf.2009.04.015. Epub 2009 Apr 21. Microbes Infect. 2009. PMID: 19389482 Free PMC article.
References
-
- Kaufmann S. Towards new leprosy and tuberculosis vaccines. Microbiol Sci. 1987;4:324–8. - PubMed
-
- Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. - PubMed
-
- Young LS, Inderlied B, Berlin OG, Gottlieb MS. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986;8:1024–33. - PubMed
-
- Canetti G. The tubercle bacillus in the pulmonary lesion in man. New York: Springer Publishing Co.; 1955.
-
- Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

