A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults
- PMID: 17559990
- PMCID: PMC2063441
- DOI: 10.1016/j.vaccine.2007.05.002
A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults
Abstract
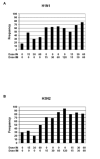
Epidemic influenza occurs annually throughout the world and is accompanied by excess morbidity and mortality. Increasing the antigen content and topical administration of vaccine are two strategies being explored to improve the immune responses to trivalent inactivated influenza vaccine (TIV). We conducted a randomized, double-blind, placebo-controlled trial to compare the immunogenicity and reactogenicity of intramuscular (IM), intranasal (IN), or combined IM and IN administration of a contemporary US vaccine formulation at escalating dosage levels in young healthy adults. Two hundred forty three healthy adults between the ages of 18 and 45 years received 15, 30, or 60mcg of trivalent inactivated influenza vaccine by either IN, IM or both routes, 120mcg of vaccine IM, or placebo IN and IM. All dosages and routes of vaccine administration were well-tolerated. A bad taste and mild nasal discomfort were more likely to be reported when influenza vaccine was administered IN, while arm tenderness was more common after IM administration. Significant increases in geometric mean serum antibody titers in both HAI and Nt assays were seen in all of the groups receiving influenza vaccine for all test antigens (P<or=.025, paired t-test), except for the B HAI antibody titer in the group that received 30mcg IN (P=.055, paired t-test). Postvaccination geometric mean serum antibody titers, the frequency of seroresponses, and the percentage achieving postvaccination serum HAI antibody titers of >or=32 were higher following delivery of the study vaccines by an IM route than by the IN route, but significant increases in serum antibody were seen after IN vaccination. Nasal IgA antibody responses were more common when vaccine was administered IN; and, when the IN dosage was increased, the primary benefit from IN vaccine over IM vaccine appeared to be greater induction of nasal secretory antibody.
Figures
Similar articles
-
Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers.Pediatrics. 2006 Sep;118(3):e579-85. doi: 10.1542/peds.2006-0201. Pediatrics. 2006. PMID: 16950949 Clinical Trial.
-
Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone®) administered by intradermal and intramuscular route in healthy adults.Vaccine. 2011 Aug 5;29(34):5666-74. doi: 10.1016/j.vaccine.2011.06.010. Epub 2011 Jun 23. Vaccine. 2011. PMID: 21699951 Free PMC article. Clinical Trial.
-
Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™).Vaccine. 2018 Jun 18;36(26):3779-3788. doi: 10.1016/j.vaccine.2018.05.053. Epub 2018 May 17. Vaccine. 2018. PMID: 29779922 Clinical Trial.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Intranasal Administration of Whole Inactivated Influenza Virus Vaccine as a Promising Influenza Vaccine Candidate.Viral Immunol. 2017 Jul/Aug;30(6):451-462. doi: 10.1089/vim.2017.0022. Epub 2017 Jun 26. Viral Immunol. 2017. PMID: 28650274 Review.
Cited by
-
Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons.J Infect Dis. 2012 Sep 15;206(6):811-20. doi: 10.1093/infdis/jis427. Epub 2012 Jul 10. J Infect Dis. 2012. PMID: 22782949 Free PMC article. Clinical Trial.
-
Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1.Vaccine. 2010 Oct 4;28(42):6901-14. doi: 10.1016/j.vaccine.2010.08.006. Epub 2010 Aug 17. Vaccine. 2010. PMID: 20723629 Free PMC article.
-
A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults.J Infect Dis. 2009 Mar 1;199(5):711-6. doi: 10.1086/596558. J Infect Dis. 2009. PMID: 19210163 Free PMC article. Clinical Trial.
-
Vaccination of lambs against Haemonchus contortus with the recombinant rHc23. Effect of adjuvant and antigen dose.PLoS One. 2018 Mar 7;13(3):e0193118. doi: 10.1371/journal.pone.0193118. eCollection 2018. PLoS One. 2018. PMID: 29513692 Free PMC article.
-
Intranasal administration of dsRNA analog poly(I:C) induces interferon-α receptor-dependent accumulation of antigen experienced T cells in the airways.PLoS One. 2012;7(12):e51351. doi: 10.1371/journal.pone.0051351. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236482 Free PMC article.
References
-
- Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–42. - PubMed
-
- Barker WH, Mullooly JP. Influenza vaccination of elderly persons. Reduction in pneumonia and influenza hospitalizations and deaths. JAMA. 1980;244:2547–9. - PubMed
-
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–7. - PubMed
-
- Keitel WA, Couch RB. Inactivated influenza vaccines. In: Potter CW, editor. Influenza. New York: Elsevier; 2002. pp. 145–77.
-
- Gross PA, Quinnan GV, Jr, Weksler ME, Gaerlan PF, Denning CR. Immunization of elderly people with high doses of influenza vaccine. J Am Geriatr Soc. 1988;36:209–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous