Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo
- PMID: 17554007
- PMCID: PMC6672150
- DOI: 10.1523/JNEUROSCI.5381-06.2007
Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo
Abstract
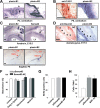
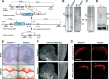
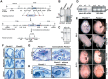
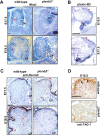
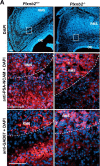
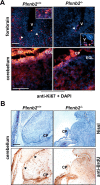
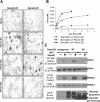
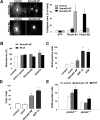
Semaphorins and their receptors, plexins, have emerged as important cellular cues regulating key developmental processes. B-type plexins directly regulate the actin cytoskeleton in a variety of cell types. Recently, B-type plexins have been shown to be expressed in striking patterns in the nervous system over critical developmental windows. However, in contrast to the well characterized plexin-A family, the functional role of plexin-B proteins in neural development and organogenesis in vertebrates in vivo is not known. Here, we have elucidated the functional contribution of the two neuronally expressed plexin-B proteins, Plexin-B1 or Plexin-B2, toward the development of the peripheral nervous system and the CNS by generating and analyzing constitutive knock-out mice. The development of the nervous system was found to be normal in mice lacking Plexin-B1, whereas mice lacking Plexin-B2 demonstrated defects in closure of the neural tube and a conspicuous disorganization of the embryonic brain. After analyzing mutant mice, which bypassed neural tube defects, we observed a key requirement for Plexin-B2 in proliferation and migration of granule cell precursors in the developing dentate gyrus, olfactory bulb, and cerebellum. Furthermore, we identified semaphorin 4C as a high-affinity ligand for Plexin-B2 in binding and functional assays. Semaphorin 4C stimulated activation of ErbB-2 and RhoA via Plexin-B2 and enhanced proliferation and migration of granule cell precursors. Semaphorin 4C-induced proliferation of ventricular zone neuroblasts was abrogated in mice lacking Plexin-B2. These genetic and functional analyses reveal a key requirement for Plexin-B2, but not Plexin-B1, in patterning of the vertebrate nervous system in vivo.
Figures


Similar articles
-
Semaphorin 4C and 4G are ligands of Plexin-B2 required in cerebellar development.Mol Cell Neurosci. 2011 Feb;46(2):419-31. doi: 10.1016/j.mcn.2010.11.005. Epub 2010 Nov 29. Mol Cell Neurosci. 2011. PMID: 21122816 Free PMC article.
-
Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis.Mol Cell Biol. 2010 Feb;30(3):764-80. doi: 10.1128/MCB.01458-09. Epub 2009 Nov 30. Mol Cell Biol. 2010. PMID: 19948886 Free PMC article.
-
Plexin-B2 controls the development of cerebellar granule cells.J Neurosci. 2007 Apr 4;27(14):3921-32. doi: 10.1523/JNEUROSCI.4710-06.2007. J Neurosci. 2007. PMID: 17409257 Free PMC article.
-
The Plexin-B family and its role in cancer progression.Histol Histopathol. 2014 Feb;29(2):151-65. doi: 10.14670/HH-29.151. Epub 2013 Sep 17. Histol Histopathol. 2014. PMID: 24043639 Review.
-
Plexin-mediated neuronal development and neuroinflammatory responses in the nervous system.Histol Histopathol. 2023 Nov;38(11):1239-1248. doi: 10.14670/HH-18-625. Epub 2023 Apr 25. Histol Histopathol. 2023. PMID: 37170703 Review.
Cited by
-
ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis.J Clin Invest. 2012 Apr;122(4):1296-305. doi: 10.1172/JCI60568. Epub 2012 Mar 1. J Clin Invest. 2012. PMID: 22378040 Free PMC article.
-
Rnd3-induced cell rounding requires interaction with Plexin-B2.J Cell Sci. 2016 Nov 1;129(21):4046-4056. doi: 10.1242/jcs.192211. Epub 2016 Sep 21. J Cell Sci. 2016. PMID: 27656111 Free PMC article.
-
The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function.Immunity. 2012 Aug 24;37(2):314-25. doi: 10.1016/j.immuni.2012.05.026. Epub 2012 Aug 16. Immunity. 2012. PMID: 22902232 Free PMC article.
-
An ENU-mutagenesis screen in the mouse: identification of novel developmental gene functions.PLoS One. 2011 Apr 29;6(4):e19357. doi: 10.1371/journal.pone.0019357. PLoS One. 2011. PMID: 21559415 Free PMC article.
-
Genetic compensation in a stable slc25a46 mutant zebrafish: A case for using F0 CRISPR mutagenesis to study phenotypes caused by inherited disease.PLoS One. 2020 Mar 24;15(3):e0230566. doi: 10.1371/journal.pone.0230566. eCollection 2020. PLoS One. 2020. PMID: 32208444 Free PMC article.
References
-
- Anderson LL, Jeftinija S, Scanes CG. Growth hormone secretion: molecular and cellular mechanisms and in vivo approaches. Exp Biol Med (Maywood) 2004;229:291–302. - PubMed
-
- Artigiani S, Barberis D, Fazzari P, Longati P, Angelini P, Van De Loo JW, Comoglio PM, Tamagnone L. Functional regulation of semaphorin receptors by proprotein convertases. J Biol Chem. 2003;278:10094–10101. - PubMed
-
- Ayoob JC, Terman JR, Kolodkin AL. Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development. 2006;133:2125–2135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

