Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia
- PMID: 17540169
- PMCID: PMC4647864
- DOI: 10.1016/j.cell.2007.03.043
Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia
Abstract
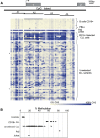
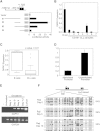
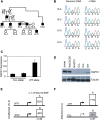
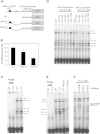
The heritability of B cell chronic lymphocytic leukemia (CLL) is relatively high; however, no predisposing mutation has been convincingly identified. We show that loss or reduced expression of death-associated protein kinase 1 (DAPK1) underlies cases of heritable predisposition to CLL and the majority of sporadic CLL. Epigenetic silencing of DAPK1 by promoter methylation occurs in almost all sporadic CLL cases. Furthermore, we defined a disease haplotype, which segregates with the CLL phenotype in a large family. DAPK1 expression of the CLL allele is downregulated by 75% in germline cells due to increased HOXB7 binding. In the blood cells from affected family members, promoter methylation results in additional loss of DAPK1 expression. Thus, reduced expression of DAPK1 can result from germline predisposition, as well as epigenetic or somatic events causing or contributing to the CLL phenotype.
Figures



Comment in
-
Chronic lymphocytic leukemia: keeping cell death at bay.Cell. 2007 Jun 1;129(5):853-5. doi: 10.1016/j.cell.2007.05.023. Cell. 2007. PMID: 17540163
Similar articles
-
Germline allele-specific expression of DAPK1 in chronic lymphocytic leukemia.PLoS One. 2013;8(1):e55261. doi: 10.1371/journal.pone.0055261. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23383130 Free PMC article.
-
Chronic lymphocytic leukemia: keeping cell death at bay.Cell. 2007 Jun 1;129(5):853-5. doi: 10.1016/j.cell.2007.05.023. Cell. 2007. PMID: 17540163
-
A noncanonical Flt3ITD/NF-κB signaling pathway represses DAPK1 in acute myeloid leukemia.Clin Cancer Res. 2012 Jan 15;18(2):360-369. doi: 10.1158/1078-0432.CCR-10-3022. Epub 2011 Nov 17. Clin Cancer Res. 2012. PMID: 22096027 Free PMC article.
-
DNA methylation, epimutations and cancer predisposition.Int J Biochem Cell Biol. 2009 Jan;41(1):34-9. doi: 10.1016/j.biocel.2008.09.006. Epub 2008 Sep 16. Int J Biochem Cell Biol. 2009. PMID: 18835361 Review.
-
Molecular profiling of chronic lymphocytic leukaemia: genetics meets epigenetics to identify predisposing genes.Br J Haematol. 2007 Dec;139(5):744-52. doi: 10.1111/j.1365-2141.2007.06875.x. Epub 2007 Oct 24. Br J Haematol. 2007. PMID: 17961188 Review.
Cited by
-
Global promoter methylation analysis reveals novel candidate tumor suppressor genes in natural killer cell lymphoma.Clin Cancer Res. 2015 Apr 1;21(7):1699-711. doi: 10.1158/1078-0432.CCR-14-1216. Epub 2015 Jan 22. Clin Cancer Res. 2015. PMID: 25614448 Free PMC article.
-
Role of NOXA and its ubiquitination in proteasome inhibitor-induced apoptosis in chronic lymphocytic leukemia cells.Haematologica. 2010 Sep;95(9):1510-8. doi: 10.3324/haematol.2010.022368. Epub 2010 Apr 7. Haematologica. 2010. PMID: 20378569 Free PMC article.
-
A high-density SNP genome-wide linkage search of 206 families identifies susceptibility loci for chronic lymphocytic leukemia.Blood. 2007 Nov 1;110(9):3326-33. doi: 10.1182/blood-2007-05-091561. Epub 2007 Aug 8. Blood. 2007. PMID: 17687107 Free PMC article.
-
Roco Proteins and the Parkinson's Disease-Associated LRRK2.Int J Mol Sci. 2018 Dec 17;19(12):4074. doi: 10.3390/ijms19124074. Int J Mol Sci. 2018. PMID: 30562929 Free PMC article. Review.
-
Cis-Acting Factors Causing Secondary Epimutations: Impact on the Risk for Cancer and Other Diseases.Cancers (Basel). 2021 Sep 26;13(19):4807. doi: 10.3390/cancers13194807. Cancers (Basel). 2021. PMID: 34638292 Free PMC article. Review.
References
-
- Amundadottir L.T., Thorvaldsson S., Gudbjartsson D.F., Sulem P., Kristjansson K., Arnason S., Gulcher J.R., Bjornsson J., Kong A., Thorsteinsdottir U., Stefansson K. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 2004;1:e65. - PMC - PubMed
-
- Bialik S., Kimchi A. The Death-Associated Protein Kinases: Structure, Function, and Beyond. Annu. Rev. Biochem. 2006;75:189–200. - PubMed
-
- Bullrich F., Fujii H., Calin G., Mabuchi H., Negrini M., Pekarsky Y., Rassenti L., Alder H., Reed J.C., Keating M.J. Characterization of the 13q14 tumor suppressor locus in CLL: identification of ALT1, an alternative splice variant of the LEU2 gene. Cancer Res. 2001;61:6640–6648. - PubMed
-
- Calin G.A., Croce C.M. Genomics of chronic lymphocytic leukemia microRNAs as new players with clinical significance. Semin. Oncol. 2006;33:167–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

