CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease
- PMID: 17525800
- PMCID: PMC1868786
- DOI: 10.1172/JCI30504
CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease
Abstract
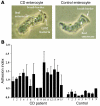
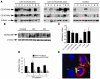
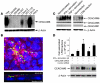
The ileal mucosa of Crohn disease (CD) patients is abnormally colonized by adherent-invasive E. coli (AIEC) that are able to adhere to and invade intestinal epithelial cells. Here, we show that CD-associated AIEC strains adhere to the brush border of primary ileal enterocytes isolated from CD patients but not controls without inflammatory bowel disease. AIEC adhesion is dependent on type 1 pili expression on the bacterial surface and on carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) expression on the apical surface of ileal epithelial cells. We report also that CEACAM6 acts as a receptor for AIEC adhesion and is abnormally expressed by ileal epithelial cells in CD patients. In addition, our in vitro studies show that there is increased CEACAM6 expression in cultured intestinal epithelial cells after IFN-gamma or TNF-alpha stimulation and after infection with AIEC bacteria, indicating that AIEC can promote its own colonization in CD patients.
Figures
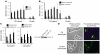

Similar articles
-
Abnormal CEACAM6 expression in Crohn disease patients favors gut colonization and inflammation by adherent-invasive E. coli.Virulence. 2010 Jul-Aug;1(4):281-2. doi: 10.4161/viru.1.4.11510. Virulence. 2010. PMID: 21178454
-
Crohn disease-associated Escherichia coli promote gastrointestinal inflammatory disorders by activation of HIF-dependent responses.Gut Microbes. 2011 Nov-Dec;2(6):335-46. doi: 10.4161/gmic.18771. Epub 2011 Nov 1. Gut Microbes. 2011. PMID: 22157238
-
Development of Heptylmannoside-Based Glycoconjugate Antiadhesive Compounds against Adherent-Invasive Escherichia coli Bacteria Associated with Crohn's Disease.mBio. 2015 Nov 17;6(6):e01298-15. doi: 10.1128/mBio.01298-15. mBio. 2015. PMID: 26578673 Free PMC article.
-
E. coli-mediated gut inflammation in genetically predisposed Crohn's disease patients.Pathol Biol (Paris). 2013 Oct;61(5):e65-9. doi: 10.1016/j.patbio.2010.01.004. Epub 2010 Apr 8. Pathol Biol (Paris). 2013. PMID: 20381273 Review.
-
Pathogenic agents in inflammatory bowel diseases.Curr Opin Gastroenterol. 2008 Jul;24(4):440-7. doi: 10.1097/MOG.0b013e3283023be5. Curr Opin Gastroenterol. 2008. PMID: 18622157 Review.
Cited by
-
Host-pathobiont interactions in Crohn's disease.Nat Rev Gastroenterol Hepatol. 2024 Oct 24. doi: 10.1038/s41575-024-00997-y. Online ahead of print. Nat Rev Gastroenterol Hepatol. 2024. PMID: 39448837 Review.
-
The JAK inhibitor, Tofacitinib, Corrects the Overexpression of CEACAM6 and Limits Susceptibility to AIEC Caused by Reduced Activity of the IBD Associated Gene, PTPN2.medRxiv [Preprint]. 2024 Sep 28:2024.09.26.24314341. doi: 10.1101/2024.09.26.24314341. medRxiv. 2024. PMID: 39399045 Free PMC article. Preprint.
-
The Role of Propionate-Induced Rearrangement of Membrane Proteins in the Formation of the Virulent Phenotype of Crohn's Disease-Associated Adherent-Invasive Escherichia coli.Int J Mol Sci. 2024 Sep 20;25(18):10118. doi: 10.3390/ijms251810118. Int J Mol Sci. 2024. PMID: 39337603 Free PMC article.
-
Towards Understanding Tumour Colonisation by Probiotic Bacterium E. coli Nissle 1917.Cancers (Basel). 2024 Aug 26;16(17):2971. doi: 10.3390/cancers16172971. Cancers (Basel). 2024. PMID: 39272829 Free PMC article. Review.
-
A single rare σ70 variant establishes a unique gene expression pattern in the E. coli pathobiont LF82.Nucleic Acids Res. 2024 Oct 28;52(19):11552-11570. doi: 10.1093/nar/gkae773. Nucleic Acids Res. 2024. PMID: 39258538 Free PMC article.
References
-
- Loftus E.V., Jr., et al. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. - PubMed
-
- Elson C.O. Commensal bacteria as targets in Crohn’s disease. Gastroenterology. 2000;119:254–257. - PubMed
-
- Podolsky D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002;347:417–429. - PubMed
-
- Shanahan F. Crohn’s disease. Lancet. 2002;359:62–69. - PubMed
-
- Hugot J.P., et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

