Alterations in xenobiotic metabolism in the long-lived Little mice
- PMID: 17521389
- PMCID: PMC2859448
- DOI: 10.1111/j.1474-9726.2007.00300.x
Alterations in xenobiotic metabolism in the long-lived Little mice
Abstract
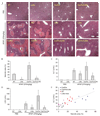
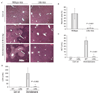
Our previous microarray expression analysis of the long-lived Little mice (Ghrhr(lit/lit)) showed a concerted up-regulation of xenobiotic detoxification genes. Here, we show that this up-regulation is associated with a potent increase in resistance against the adverse effects of a variety of xenobiotics, including the hepatotoxins acetaminophen and bromobenzene and the paralyzing agent zoxazolamine. The classic xenobiotic receptors Car (Constitutive Androstane Receptor) and Pxr (Pregnane X Receptor) are considered key regulators of xenobiotic metabolism. Using double and triple knockout/mutant mouse models we found, however, that Car and Pxr are not required for the up-regulation of xenobiotic genes in Little mice. Our results suggest instead that bile acids and the primary bile acid receptor Fxr (farnesoid X receptor) are likely mediators of the up-regulation of xenobiotic detoxification genes in Little mice. Bile acid levels are considerably elevated in the bile, serum, and liver of Little mice. We found that treatment of wild-type animals with cholic acid, one of the major bile acids elevated in Little mice, mimics in large part the up-regulation of xenobiotic detoxification genes observed in Little mice. Additionally, the loss of Fxr had a major effect on the expression of the xenobiotic detoxification genes up-regulated in Little mice. A large fraction of these genes lost or decreased their high expression levels in double mutant mice for Fxr and Ghrhr. The alterations in xenobiotic metabolism in Little mice constitute a form of increased stress resistance and may contribute to the extended longevity of these mice.
Figures




Comment in
-
Long-lived dwarf mice: are bile acids a longevity signal?Aging Cell. 2007 Aug;6(4):421-3. doi: 10.1111/j.1474-9726.2007.00309.x. Aging Cell. 2007. PMID: 17578511
Similar articles
-
Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury.Proc Natl Acad Sci U S A. 2005 Feb 8;102(6):2063-8. doi: 10.1073/pnas.0409794102. Epub 2005 Jan 31. Proc Natl Acad Sci U S A. 2005. PMID: 15684063 Free PMC article.
-
The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors.Mol Endocrinol. 2007 Jul;21(7):1603-16. doi: 10.1210/me.2007-0133. Epub 2007 Apr 24. Mol Endocrinol. 2007. PMID: 17456796
-
Pregnane X receptor is a target of farnesoid X receptor.J Biol Chem. 2006 Jul 14;281(28):19081-91. doi: 10.1074/jbc.M600116200. Epub 2006 May 8. J Biol Chem. 2006. PMID: 16682417
-
CAR and PXR: xenosensors of endocrine disrupters?Chem Biol Interact. 2005 Aug 15;155(3):111-28. doi: 10.1016/j.cbi.2005.06.003. Chem Biol Interact. 2005. PMID: 16054614 Review.
-
FXR: a metabolic regulator and cell protector.Cell Res. 2008 Nov;18(11):1087-95. doi: 10.1038/cr.2008.289. Cell Res. 2008. PMID: 18825165 Review.
Cited by
-
Rapamycin slows aging in mice.Aging Cell. 2012 Aug;11(4):675-82. doi: 10.1111/j.1474-9726.2012.00832.x. Epub 2012 Jun 4. Aging Cell. 2012. PMID: 22587563 Free PMC article.
-
Microsomal quercetin glucuronidation in rat small intestine depends on age and segment.Drug Metab Dispos. 2011 Aug;39(8):1406-14. doi: 10.1124/dmd.111.038406. Epub 2011 May 4. Drug Metab Dispos. 2011. PMID: 21543555 Free PMC article.
-
Nuclear hormone receptor DHR96 mediates the resistance to xenobiotics but not the increased lifespan of insulin-mutant Drosophila.Proc Natl Acad Sci U S A. 2016 Feb 2;113(5):1321-6. doi: 10.1073/pnas.1515137113. Epub 2016 Jan 19. Proc Natl Acad Sci U S A. 2016. PMID: 26787908 Free PMC article.
-
Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice.BMB Rep. 2016 Feb;49(2):105-10. doi: 10.5483/bmbrep.2016.49.2.173. BMB Rep. 2016. PMID: 26350747 Free PMC article.
-
Xenohormetic, hormetic and cytostatic selective forces driving longevity at the ecosystemic level.Aging (Albany NY). 2010 Aug;2(8):461-70. doi: 10.18632/aging.100186. Aging (Albany NY). 2010. PMID: 20693605 Free PMC article.
References
-
- Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. - PubMed
-
- Alvaro D, Gigliozzi A, Attili AF. Regulation and deregulation of cholangiocyte proliferation. J. Hepatol. 2000;33:333–340. - PubMed
-
- Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. - PubMed
-
- Ansher SS, Dolan P, Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983;3:932–935. - PubMed
-
- Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, De Castri D, Moscheni C, Annoni G. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol. Toxicol. 2000;87:229–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

