Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells
- PMID: 17513769
- PMCID: PMC2806821
- DOI: 10.4049/jimmunol.178.11.7199
Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells
Abstract
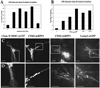
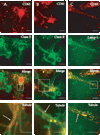
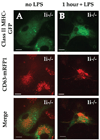
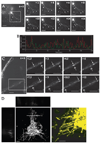
Immature dendritic cells (DCs) capture exogenous Ags in the periphery for eventual processing in endolysosomes. Upon maturation by TLR agonists, DCs deliver peptide-loaded class II MHC molecules from these compartments to the cell surface via long tubular structures (endolysosomal tubules). The nature and rules that govern the movement of these DC compartments are unknown. In this study, we demonstrate that the tubules contain multiple proteins including the class II MHC molecules and LAMP1, a lysosomal resident protein, as well as CD63 and CD82, members of the tetraspanin family. Endolysosomal tubules can be stained with acidotropic dyes, indicating that they are extensions of lysosomes. However, the proper trafficking of class II MHC molecules themselves is not necessary for endolysosomal tubule formation. DCs lacking MyD88 can also form endolysosomal tubules, demonstrating that MyD88-dependent TLR activation is not necessary for the formation of this compartment. Endolysosomal tubules in DCs exhibit dynamic and saltatory movement, including bidirectional travel. Measured velocities are consistent with motor-based movement along microtubules. Indeed, nocodazole causes the collapse of endolysosomal tubules. In addition to its association with microtubules, endolysosomal tubules follow the plus ends of microtubules as visualized in primary DCs expressing end binding protein 1 (EB1)-enhanced GFP.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures



Similar articles
-
Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane.Nature. 2002 Aug 29;418(6901):988-94. doi: 10.1038/nature01006. Nature. 2002. PMID: 12198549
-
T cells induce extended class II MHC compartments in dendritic cells in a Toll-like receptor-dependent manner.J Immunol. 2003 Oct 15;171(8):4081-8. doi: 10.4049/jimmunol.171.8.4081. J Immunol. 2003. PMID: 14530329
-
CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells.Blood. 2004 Aug 15;104(4):1183-90. doi: 10.1182/blood-2004-01-0104. Epub 2004 May 6. Blood. 2004. PMID: 15130945
-
The labyrinth unfolds: architectural rearrangements of the endolysosomal system in antigen-presenting cells.Curr Opin Immunol. 2019 Jun;58:1-8. doi: 10.1016/j.coi.2018.12.004. Epub 2019 Feb 7. Curr Opin Immunol. 2019. PMID: 30738283 Review.
-
Membrane specializations and endosome maturation in dendritic cells and B cells.Trends Cell Biol. 2004 Apr;14(4):175-83. doi: 10.1016/j.tcb.2004.02.004. Trends Cell Biol. 2004. PMID: 15066635 Review.
Cited by
-
The Lysosome Signaling Platform: Adapting With the Times.Front Cell Dev Biol. 2019 Jun 20;7:113. doi: 10.3389/fcell.2019.00113. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31281815 Free PMC article. Review.
-
Ubiquitin-dependent control of class II MHC localization is dispensable for antigen presentation and antibody production.PLoS One. 2011 Apr 20;6(4):e18817. doi: 10.1371/journal.pone.0018817. PLoS One. 2011. PMID: 21533087 Free PMC article.
-
Dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and endoplasmic reticulum (ER) protein quality control.J Biol Chem. 2011 Oct 28;286(43):37602-14. doi: 10.1074/jbc.M111.284794. Epub 2011 Aug 20. J Biol Chem. 2011. PMID: 21857022 Free PMC article.
-
Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing β-1,3 Glucan.J Immunol. 2016 Mar 1;196(5):2249-61. doi: 10.4049/jimmunol.1401545. Epub 2016 Feb 1. J Immunol. 2016. PMID: 26829985 Free PMC article.
-
Salmonella actively modulates TFEB in murine macrophages in a growth-phase and time-dependent manner.Microbiol Spectr. 2024 Jan 11;12(1):e0498122. doi: 10.1128/spectrum.04981-22. Epub 2023 Dec 5. Microbiol Spectr. 2024. PMID: 38051049 Free PMC article.
References
-
- Boes M, Cuvillier A, Ploegh H. Membrane specializations and endosome maturation in dendritic cells and B cells. Trends Cell Biol. 2004;14:175–183. - PubMed
-
- Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 2006;7:1029–1035. - PubMed
-
- Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. - PubMed
-
- Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. - PubMed
-
- Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Curr. Opin. Immunol. 2004;16:96–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

