Gliogenesis and glial pathology in depression
- PMID: 17511618
- PMCID: PMC2918806
- DOI: 10.2174/187152707780619326
Gliogenesis and glial pathology in depression
Abstract
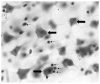
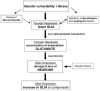
Recent research has changed the perception of glia from being no more than silent supportive cells of neurons to being dynamic partners participating in brain metabolism and communication between neurons. This discovery of new glial functions coincides with growing evidence of the involvement of glia in the neuropathology of mood disorders. Unanticipated reductions in the density and number of glial cells are reported in fronto-limbic brain regions in major depression and bipolar illness. Moreover, age-dependent decreases in the density of glial fibrillary acidic protein (GFAP) - immunoreactive astrocytes and levels of GFAP protein are observed in the prefrontal cortex of younger depressed subjects. Since astrocytes participate in the uptake, metabolism and recycling of glutamate, we hypothesize that an astrocytic deficit may account for the alterations in glutamate/GABA neurotransmission in depression. Reductions in the density and ultrastructure of oligodendrocytes are also detected in the prefrontal cortex and amygdala in depression. Pathological changes in oligodendrocytes may be relevant to the disruption of white matter tracts in mood disorders reported by diffusion tensor imaging. Factors such as stress, excess of glucocorticoids, altered gene expression of neurotrophic factors and glial transporters, and changes in extracellular levels of neurotransmitters released by neurons may modify glial cell number and affect the neurophysiology of depression. Therefore, we will explore the role of these events in the possible alteration of glial number and activity, and the capacity of glia as a promising new target for therapeutic medications. Finally, we will consider the temporal relationship between glial and neuronal cell pathology in depression.
Figures


Similar articles
-
Region specific decrease in glial fibrillary acidic protein immunoreactivity in the brain of a rat model of depression.Neuroscience. 2009 Mar 17;159(2):915-25. doi: 10.1016/j.neuroscience.2008.10.018. Epub 2008 Nov 1. Neuroscience. 2009. PMID: 19000745
-
Glial cells as key elements in the pathophysiology and treatment of bipolar disorder.Acta Neuropsychiatr. 2017 Jun;29(3):140-152. doi: 10.1017/neu.2016.56. Epub 2016 Oct 24. Acta Neuropsychiatr. 2017. PMID: 27772534 Review.
-
Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes.Biol Psychiatry. 2004 Mar 15;55(6):563-9. doi: 10.1016/j.biopsych.2003.11.006. Biol Psychiatry. 2004. PMID: 15013824
-
Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder.Cereb Cortex. 2002 Apr;12(4):386-94. doi: 10.1093/cercor/12.4.386. Cereb Cortex. 2002. PMID: 11884354
-
Altered glial plasticity in animal models for mood disorders.Curr Drug Targets. 2013 Oct;14(11):1249-61. doi: 10.2174/1389450111314110005. Curr Drug Targets. 2013. PMID: 23597041 Review.
Cited by
-
Second messenger/signal transduction pathways in major mood disorders: moving from membrane to mechanism of action, part I: major depressive disorder.CNS Spectr. 2013 Oct;18(5):231-41. doi: 10.1017/S1092852913000059. Epub 2013 Mar 5. CNS Spectr. 2013. PMID: 23462230 Free PMC article. Review.
-
Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula.Front Syst Neurosci. 2010 Jul 15;4:33. doi: 10.3389/fnsys.2010.00033. eCollection 2010. Front Syst Neurosci. 2010. PMID: 20700385 Free PMC article.
-
Astrocytes as Context for the Involvement of Myelin and Nodes of Ranvier in the Pathophysiology of Depression and Stress-Related Disorders.J Psychiatr Brain Sci. 2023;8:e230001. doi: 10.20900/jpbs.20230001. Epub 2023 Feb 22. J Psychiatr Brain Sci. 2023. PMID: 36866235 Free PMC article.
-
Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder.Neuropsychopharmacology. 2020 Mar;45(4):703-712. doi: 10.1038/s41386-019-0563-9. Epub 2019 Nov 6. Neuropsychopharmacology. 2020. PMID: 31694045 Free PMC article.
-
Beyond New Neurons in the Adult Hippocampus: Imipramine Acts as a Pro-Astrogliogenic Factor and Rescues Cognitive Impairments Induced by Stress Exposure.Cells. 2022 Jan 24;11(3):390. doi: 10.3390/cells11030390. Cells. 2022. PMID: 35159199 Free PMC article.
References
-
- Kettenmann H, Ransom BR. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 1.
-
- Volterra A, Meldolesi J. Nat. Rev. Neurosci. 2005;6:626. - PubMed
-
- Seifert G, Schilling K, Steinhauser C. Nat. Rev. Neurosci. 2006;7:194. - PubMed
-
- Rajkowska G. The Neuroscientist. 2003;9:273. - PubMed
-
- Kandel ER. In: Principles of neuronal science. 4th ed. Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill; 2000.
Publication types
MeSH terms
Grants and funding
- P50 MH060451/MH/NIMH NIH HHS/United States
- MH60451/MH/NIMH NIH HHS/United States
- R01 MH061578/MH/NIMH NIH HHS/United States
- P20 RR017701-049007/RR/NCRR NIH HHS/United States
- MH67996/MH/NIMH NIH HHS/United States
- R01 MH063187/MH/NIMH NIH HHS/United States
- R01 MH063187-04/MH/NIMH NIH HHS/United States
- P20 RR017701/RR/NCRR NIH HHS/United States
- R01 MH061578-03/MH/NIMH NIH HHS/United States
- RR17701/RR/NCRR NIH HHS/United States
- MH61578/MH/NIMH NIH HHS/United States
- R01 MH067996-04/MH/NIMH NIH HHS/United States
- MH63187/MH/NIMH NIH HHS/United States
- P50 MH060451-03/MH/NIMH NIH HHS/United States
- R01 MH067996/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
