An Acanthamoeba sp. containing two phylogenetically different bacterial endosymbionts
- PMID: 17504498
- PMCID: PMC1974821
- DOI: 10.1111/j.1462-2920.2007.01268.x
An Acanthamoeba sp. containing two phylogenetically different bacterial endosymbionts
Abstract
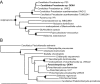
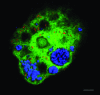
Acanthamoebae are ubiquitous free-living amoebae and important predators of microbial communities. They frequently contain obligate intracellular bacterial symbionts, which show a worldwide distribution. All Acanthamoeba spp. described so far harboured no or only a single specific endosymbiont phylotype, and in some cases evidence for coevolution between the symbiotic bacteria and the amoeba host has been reported. In this study we have isolated and characterized an Acanthamoeba sp. (strain OEW1) showing a stable symbiotic relationship with two morphologically different endosymbionts. 16S rRNA sequence analysis assigned these symbionts to the candidate genus Procabacter (Betaproteobacteria) and the genus Parachlamydia (Chlamydiae) respectively. Fluorescence in situ hybridization and transmission electron microscopy confirmed the affiliation of the endosymbionts and showed their co-occurrence in the amoeba host cells and their intracellular location within separate compartments enclosed by host-derived membranes. Further analysis of this stable relationship should provide novel insights into the complex interactions of intracellular multiple-partner associations.
Figures

Similar articles
-
Obligate bacterial endosymbionts of Acanthamoeba spp. related to the beta-Proteobacteria: proposal of 'Candidatus Procabacter acanthamoebae' gen. nov., sp. nov.Int J Syst Evol Microbiol. 2002 Mar;52(Pt 2):599-605. doi: 10.1099/00207713-52-2-599. Int J Syst Evol Microbiol. 2002. PMID: 11931173
-
Novel Chlamydiales strains isolated from a water treatment plant.Environ Microbiol. 2009 Jan;11(1):188-200. doi: 10.1111/j.1462-2920.2008.01752.x. Epub 2008 Sep 12. Environ Microbiol. 2009. PMID: 18793313
-
Saccamoeba lacustris, sp. nov. (Amoebozoa: Lobosea: Hartmannellidae), a new lobose amoeba, parasitized by the novel chlamydia 'Candidatus Metachlamydia lacustris' (Chlamydiae: Parachlamydiaceae).Eur J Protistol. 2010 May;46(2):86-95. doi: 10.1016/j.ejop.2009.11.002. Epub 2010 Mar 27. Eur J Protistol. 2010. PMID: 20347279
-
Bacterial endosymbionts of free-living amoebae.J Eukaryot Microbiol. 2004 Sep-Oct;51(5):509-14. doi: 10.1111/j.1550-7408.2004.tb00278.x. J Eukaryot Microbiol. 2004. PMID: 15537084 Review.
-
Parachlamydia acanthamoebae, an emerging agent of pneumonia.Clin Microbiol Infect. 2009 Jan;15(1):18-28. doi: 10.1111/j.1469-0691.2008.02633.x. Clin Microbiol Infect. 2009. PMID: 19220336 Review.
Cited by
-
Host-Associated Genomic Features of the Novel Uncultured Intracellular Pathogen Ca. Ichthyocystis Revealed by Direct Sequencing of Epitheliocysts.Genome Biol Evol. 2016 Jun 13;8(6):1672-89. doi: 10.1093/gbe/evw111. Genome Biol Evol. 2016. PMID: 27190004 Free PMC article.
-
Detection of bacterial endosymbionts in clinical acanthamoeba isolates.Ophthalmology. 2010 Mar;117(3):445-52, 452.e1-3. doi: 10.1016/j.ophtha.2009.08.033. Epub 2010 Jan 19. Ophthalmology. 2010. PMID: 20031220 Free PMC article.
-
A bacterial genome in transition--an exceptional enrichment of IS elements but lack of evidence for recent transposition in the symbiont Amoebophilus asiaticus.BMC Evol Biol. 2011 Sep 26;11:270. doi: 10.1186/1471-2148-11-270. BMC Evol Biol. 2011. PMID: 21943072 Free PMC article.
-
Massive expansion of Ubiquitination-related gene families within the Chlamydiae.Mol Biol Evol. 2014 Nov;31(11):2890-904. doi: 10.1093/molbev/msu227. Epub 2014 Jul 28. Mol Biol Evol. 2014. PMID: 25069652 Free PMC article.
-
Free-Living Amoebae as Hosts for and Vectors of Intracellular Microorganisms with Public Health Significance.Viruses. 2017 Apr 1;9(4):65. doi: 10.3390/v9040065. Viruses. 2017. PMID: 28368313 Free PMC article. Review.
References
-
- Barker J, Brown MRW. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology. 1994;140:1253–1259. - PubMed
-
- Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

