Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists
- PMID: 17502848
- PMCID: PMC2014123
- DOI: 10.1038/sj.bjp.0707277
Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists
Abstract
Background and purpose: Ultralow doses of naltrexone, a non-selective opioid antagonist, have previously been found to augment acute morphine analgesia and block the development of tolerance to this effect. Since morphine tolerance is dependent on the activity of micro and delta receptors, the present study investigated the effects of ultralow doses of antagonists selective for these receptor types on morphine analgesia and tolerance in tests of thermal and mechanical nociception.
Experimental approach: Effects of intrathecal administration of mu-receptor antagonists, CTOP (0.01 ng) or CTAP (0.001 ng), or a delta-receptor antagonist, naltrindole (0.01 ng), on spinal morphine analgesia and tolerance were evaluated using the tail-flick and paw-pressure tests in rats.
Key results: Both micro and delta antagonists augmented analgesia produced by a sub-maximal (5 microg) or maximal (15 microg) dose of morphine. Administration of the antagonists with morphine (15 microg) for 5 days inhibited the progressive decline of analgesia and prevented the loss of morphine potency. In animals exhibiting tolerance to morphine, administration of the antagonists with morphine produced a recovery of the analgesic response and restored morphine potency.
Conclusions and implications: Combining ultralow doses of micro- or delta-receptor antagonists with spinal morphine augmented the acute analgesic effects, inhibited the induction of chronic tolerance and reversed established tolerance. The remarkably similar effects of micro- and delta-opioid receptor antagonists on morphine analgesia and tolerance are interpreted in terms of blockade of the latent excitatory effects of the agonist that limit expression of its full activity.
Figures
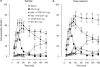
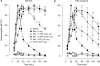
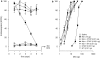
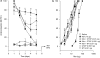
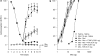
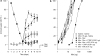
Similar articles
-
Inhibition of tolerance to spinal morphine antinociception by low doses of opioid receptor antagonists.Eur J Pharmacol. 2007 Apr 10;560(2-3):132-41. doi: 10.1016/j.ejphar.2006.12.013. Epub 2007 Jan 17. Eur J Pharmacol. 2007. PMID: 17307158
-
Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats.J Pharmacol Exp Ther. 2002 Feb;300(2):588-96. doi: 10.1124/jpet.300.2.588. J Pharmacol Exp Ther. 2002. PMID: 11805221
-
Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors.Brain Res. 2006 Apr 14;1083(1):61-9. doi: 10.1016/j.brainres.2006.01.095. Epub 2006 Mar 10. Brain Res. 2006. PMID: 16530171
-
Modulatory effects of Gs-coupled excitatory opioid receptor functions on opioid analgesia, tolerance, and dependence.Neurochem Res. 1996 Nov;21(11):1347-51. doi: 10.1007/BF02532375. Neurochem Res. 1996. PMID: 8947924 Review.
-
Antagonists of excitatory opioid receptor functions enhance morphine's analgesic potency and attenuate opioid tolerance/dependence liability.Pain. 2000 Feb;84(2-3):121-31. doi: 10.1016/s0304-3959(99)00223-7. Pain. 2000. PMID: 10666516 Review.
Cited by
-
Implication of delta opioid receptor subtype 2 but not delta opioid receptor subtype 1 in the development of morphine analgesic tolerance in a rat model of chronic inflammatory pain.Eur J Neurosci. 2015 Apr;41(7):901--7. doi: 10.1111/ejn.12829. Epub 2015 Jan 9. Eur J Neurosci. 2015. PMID: 25639561 Free PMC article.
-
An integrated quantitative proteomics and systems biology approach to explore synaptic protein profile changes during morphine exposure.Neuropsychopharmacology. 2014 Jan;39(1):88-103. doi: 10.1038/npp.2013.227. Epub 2013 Sep 18. Neuropsychopharmacology. 2014. PMID: 24045585 Free PMC article. Review.
-
Functional relevance of μ-δ opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology.Drug Alcohol Depend. 2012 Mar 1;121(3):167-72. doi: 10.1016/j.drugalcdep.2011.10.025. Epub 2011 Nov 23. Drug Alcohol Depend. 2012. PMID: 22115888 Free PMC article. Review.
-
Disease-specific heteromerization of G-protein-coupled receptors that target drugs of abuse.Prog Mol Biol Transl Sci. 2013;117:207-65. doi: 10.1016/B978-0-12-386931-9.00009-X. Prog Mol Biol Transl Sci. 2013. PMID: 23663971 Free PMC article. Review.
-
Biphasic effects of naloxone in the rats receiving morphine overdose a place preference study.Iran J Pharm Res. 2011 Summer;10(3):605-10. Iran J Pharm Res. 2011. PMID: 24250394 Free PMC article.
References
-
- Allard M, Zajac JM, Simonnet G. Autoradiographic distribution of receptors to FLFQPQRFamide, a morphine-modulating peptide, in rat central nervous system. Neuroscience. 1992;49:101–116. - PubMed
-
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance. Trends Pharmacol Sci. 2006;27:558–565. - PubMed
-
- Bonini JA, Jones KA, Adham N, Forray C, Artymyshyn R, Durkin MM, et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem. 2000;275:39324–39331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

