Stable differentiation and clonality of murine long-term hematopoiesis after extended reduced-intensity selection for MGMT P140K transgene expression
- PMID: 17496202
- PMCID: PMC1976372
- DOI: 10.1182/blood-2006-11-053710
Stable differentiation and clonality of murine long-term hematopoiesis after extended reduced-intensity selection for MGMT P140K transgene expression
Abstract
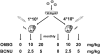
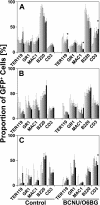
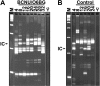
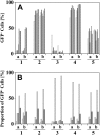
Efficient in vivo selection increases survival of gene-corrected hematopoietic stem cells (HSCs) and protects hematopoiesis, even if initial gene transfer efficiency is low. Moreover, selection of a limited number of transduced HSCs lowers the number of cell clones at risk of gene activation by insertional mutagenesis. However, a limited clonal repertoire greatly increases the proliferation stress of each individual clone. Therefore, understanding the impact of in vivo selection on proliferation and lineage differentiation of stem-cell clones is essential for its clinical use. We established minimal cell and drug dosage requirements for selection of P140K mutant O6-methylguanine-DNA-methyltransferase (MGMT P140K)-expressing HSCs and monitored their differentiation potential and clonality under long-term selective stress. Up to 17 administrations of O6-benzylguanine (O6-BG) and 1,3-bis(2-chloroethyl)-1-nitroso-urea (BCNU) did not impair long-term differentiation and proliferation of MGMT P140K-expressing stem-cell clones in mice that underwent serial transplantation and did not lead to clonal exhaustion. Interestingly, not all gene-modified hematopoietic repopulating cell clones were efficiently selectable. Our studies demonstrate that the normal function of murine hematopoietic stem and progenitor cells is not compromised by reduced-intensity long-term in vivo selection, thus underscoring the potential value of MGMT P140K selection for clinical gene therapy.
Figures
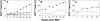

Similar articles
-
Hematopoietic expression of O(6)-methylguanine DNA methyltransferase-P140K allows intensive treatment of human glioma xenografts with combination O(6)-benzylguanine and 1,3-bis-(2-chloroethyl)-1-nitrosourea.Mol Cancer Ther. 2003 Dec;2(12):1321-9. Mol Cancer Ther. 2003. PMID: 14707273
-
Direct reversal of DNA damage by mutant methyltransferase protein protects mice against dose-intensified chemotherapy and leads to in vivo selection of hematopoietic stem cells.Cancer Res. 2000 Sep 15;60(18):5187-95. Cancer Res. 2000. PMID: 11016647
-
Characterisation of a P140K mutant O6-methylguanine-DNA-methyltransferase (MGMT)-expressing transgenic mouse line with drug-selectable bone marrow.J Gene Med. 2006 Sep;8(9):1071-85. doi: 10.1002/jgm.937. J Gene Med. 2006. PMID: 16927363
-
Vector design for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells.DNA Repair (Amst). 2007 Aug 1;6(8):1187-96. doi: 10.1016/j.dnarep.2007.03.017. Epub 2007 May 7. DNA Repair (Amst). 2007. PMID: 17482894 Free PMC article. Review.
-
Clinical relevance of MGMT in the treatment of cancer.J Clin Oncol. 2002 May 1;20(9):2388-99. doi: 10.1200/JCO.2002.06.110. J Clin Oncol. 2002. PMID: 11981013 Review.
Cited by
-
Amelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance gene.Blood. 2009 Jun 4;113(23):5747-56. doi: 10.1182/blood-2008-10-186684. Epub 2009 Apr 13. Blood. 2009. PMID: 19365082 Free PMC article.
-
Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients.Blood. 2009 May 21;113(21):5094-103. doi: 10.1182/blood-2008-09-176412. Epub 2009 Mar 31. Blood. 2009. PMID: 19336761 Free PMC article.
-
Towards in vivo amplification: Overcoming hurdles in the use of hematopoietic stem cells in transplantation and gene therapy.World J Stem Cells. 2015 Dec 26;7(11):1233-50. doi: 10.4252/wjsc.v7.i11.1233. World J Stem Cells. 2015. PMID: 26730268 Free PMC article. Review.
-
Modulation of radiation-induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy.Radiat Res. 2008 Oct;170(4):437-43. doi: 10.1667/rr1286.1. Radiat Res. 2008. PMID: 19024650 Free PMC article.
-
Cell-intrinsic and vector-related properties cooperate to determine the incidence and consequences of insertional mutagenesis.Mol Ther. 2009 Sep;17(9):1537-47. doi: 10.1038/mt.2009.134. Epub 2009 Jun 16. Mol Ther. 2009. PMID: 19532134 Free PMC article.
References
-
- Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. - PubMed
-
- Hacein-Bey-Abina S, Fischer A, Cavazzana-Calvo M. Gene therapy of X-linked severe combined immunodeficiency. Int J Hematol. 2002;76:295–298. - PubMed
-
- Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
-
- Aiuti A, Vai S, Mortellaro A, et al. Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement. Nat Med. 2002;8:423–425. - PubMed
-
- Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

