H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine
- PMID: 17468205
- PMCID: PMC2803310
- DOI: 10.1113/expphysiol.2005.029959
H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine
Abstract
The H(+)-electrochemical gradient was originally considered as a driving force for solute transport only across cellular membranes of bacteria, plants and yeast. However, in the mammalian small intestine, a H(+)-electrochemical gradient is present at the epithelial brush-border membrane in the form of an acid microclimate. Over recent years, a large number of H(+)-coupled cotransport mechanisms have been identified at the luminal membrane of the mammalian small intestine. These transporters are responsible for the initial stage in absorption of a remarkable variety of essential and non-essential nutrients and micronutrients, including protein digestion products (di/tripeptides and amino acids), vitamins, short-chain fatty acids and divalent metal ions. Proton-coupled cotransporters expressed at the mammalian small intestinal brush-border membrane include: the di/tripeptide transporter PepT1 (SLC15A1); the proton-coupled amino-acid transporter PAT1 (SLC36A1); the divalent metal transporter DMT1 (SLC11A2); the organic anion transporting polypeptide OATP2B1 (SLC02B1); the monocarboxylate transporter MCT1 (SLC16A1); the proton-coupled folate transporter PCFT (SLC46A1); the sodium-glucose linked cotransporter SGLT1 (SLC5A1); and the excitatory amino acid carrier EAAC1 (SLC1A1). Emerging research demonstrates that the optimal intestinal absorptive capacity of certain H(+)-coupled cotransporters (PepT1 and PAT1) is dependent upon function of the brush-border Na(+)-H(+) exchanger NHE3 (SLC9A3). The high oral bioavailability of a large number of pharmaceutical compounds results, in part, from absorptive transport via the same H(+)-coupled cotransporters. Drugs undergoing H(+)-coupled cotransport across the intestinal brush-border membrane include those used to treat bacterial infections, hypercholesterolaemia, hypertension, hyperglycaemia, viral infections, allergies, epilepsy, schizophrenia, rheumatoid arthritis and cancer.
Figures
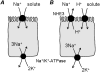
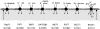
Similar articles
-
H+/amino acid transporter 1 (PAT1) is the imino acid carrier: An intestinal nutrient/drug transporter in human and rat.Gastroenterology. 2004 Nov;127(5):1410-22. doi: 10.1053/j.gastro.2004.08.017. Gastroenterology. 2004. PMID: 15521011
-
Chronic and selective inhibition of basolateral membrane Na-K-ATPase uniquely regulates brush border membrane Na absorption in intestinal epithelial cells.Am J Physiol Cell Physiol. 2015 Apr 15;308(8):C650-6. doi: 10.1152/ajpcell.00355.2014. Epub 2015 Feb 4. Am J Physiol Cell Physiol. 2015. PMID: 25652450 Free PMC article.
-
Transport of the photodynamic therapy agent 5-aminolevulinic acid by distinct H+-coupled nutrient carriers coexpressed in the small intestine.J Pharmacol Exp Ther. 2010 Jan;332(1):220-8. doi: 10.1124/jpet.109.159822. Epub 2009 Sep 29. J Pharmacol Exp Ther. 2010. PMID: 19789362 Free PMC article.
-
Hijacking solute carriers for proton-coupled drug transport.Physiology (Bethesda). 2010 Dec;25(6):364-77. doi: 10.1152/physiol.00027.2010. Physiology (Bethesda). 2010. PMID: 21186281 Review.
-
Dietary fructose, salt absorption and hypertension in metabolic syndrome: towards a new paradigm.Acta Physiol (Oxf). 2011 Jan;201(1):55-62. doi: 10.1111/j.1748-1716.2010.02167.x. Acta Physiol (Oxf). 2011. PMID: 21143427 Free PMC article. Review.
Cited by
-
Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth.Cancers (Basel). 2024 Jan 24;16(3):504. doi: 10.3390/cancers16030504. Cancers (Basel). 2024. PMID: 38339256 Free PMC article. Review.
-
VARIDT 2.0: structural variability of drug transporter.Nucleic Acids Res. 2022 Jan 7;50(D1):D1417-D1431. doi: 10.1093/nar/gkab1013. Nucleic Acids Res. 2022. PMID: 34747471 Free PMC article.
-
The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport.Br J Pharmacol. 2011 Dec;164(7):1802-16. doi: 10.1111/j.1476-5381.2011.01438.x. Br J Pharmacol. 2011. PMID: 21501141 Free PMC article. Review.
-
L-valine is a powerful stimulator of GLP-1 secretion in rodents and stimulates secretion through ATP-sensitive potassium channels and voltage-gated calcium channels.Nutr Diabetes. 2024 Jun 11;14(1):43. doi: 10.1038/s41387-024-00303-4. Nutr Diabetes. 2024. PMID: 38862477 Free PMC article.
-
siRNA capsulated brain-targeted nanoparticles specifically knock down OATP2B1 in mice: a mechanism for acute morphine tolerance suppression.Sci Rep. 2016 Sep 15;6:33338. doi: 10.1038/srep33338. Sci Rep. 2016. PMID: 27629937 Free PMC article.
References
-
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, Munck BG, Daniel H, Ganapathy V, Thwaites DT. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. - PubMed
-
- Anderson CMH, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1) J Cell Physiol. 2005;204:604–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

