Epstein-Barr virus entry
- PMID: 17459936
- PMCID: PMC1951282
- DOI: 10.1128/JVI.00445-07
Epstein-Barr virus entry
Figures

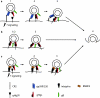
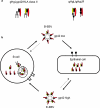
Similar articles
-
Two Epstein-Barr virus glycoprotein complexes.Curr Top Microbiol Immunol. 2001;258:51-64. doi: 10.1007/978-3-642-56515-1_4. Curr Top Microbiol Immunol. 2001. PMID: 11443867 Review. No abstract available.
-
Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8.Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20464-9. doi: 10.1073/pnas.0907508106. Epub 2009 Nov 17. Proc Natl Acad Sci U S A. 2009. PMID: 19920174 Free PMC article.
-
Epstein-Barr virus shed in saliva is high in B-cell-tropic glycoprotein gp42.J Virol. 2006 Jul;80(14):7281-3. doi: 10.1128/JVI.00497-06. J Virol. 2006. PMID: 16809335 Free PMC article.
-
Epstein-Barr virus and the germinal center B cells.Exp Hematol. 2006 Jun;34(6):695-6. doi: 10.1016/j.exphem.2006.02.021. Exp Hematol. 2006. PMID: 16728272 Review. No abstract available.
-
Viral Entry.Curr Top Microbiol Immunol. 2015;391:221-35. doi: 10.1007/978-3-319-22834-1_7. Curr Top Microbiol Immunol. 2015. PMID: 26428376 Review.
Cited by
-
Human complement receptor type 1/CD35 is an Epstein-Barr Virus receptor.Cell Rep. 2013 Feb 21;3(2):371-85. doi: 10.1016/j.celrep.2013.01.023. Epub 2013 Feb 14. Cell Rep. 2013. PMID: 23416052 Free PMC article.
-
Exploitation of Cellular Cytoskeletons and Signaling Pathways for Cell Entry by Kaposi's Sarcoma-Associated Herpesvirus and the Closely Related Rhesus Rhadinovirus.Pathogens. 2012 Dec;1(2):102-27. doi: 10.3390/pathogens1020102. Epub 2012 Oct 22. Pathogens. 2012. PMID: 23420076 Free PMC article.
-
Antibody evasion by a gammaherpesvirus O-glycan shield.PLoS Pathog. 2011 Nov;7(11):e1002387. doi: 10.1371/journal.ppat.1002387. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22114560 Free PMC article.
-
iTIME.219: An Immortalized KSHV Infected Endothelial Cell Line Inducible by a KSHV-Specific Stimulus to Transition From Latency to Lytic Replication and Infectious Virus Release.Front Cell Infect Microbiol. 2021 Apr 14;11:654396. doi: 10.3389/fcimb.2021.654396. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33937098 Free PMC article.
-
The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells.Virology. 2008 Jan 20;370(2):430-42. doi: 10.1016/j.virol.2007.09.012. Epub 2007 Oct 22. Virology. 2008. PMID: 17945327 Free PMC article.
References
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
-
- D'Addario, M., T. A. Libermann, J. Xu, A. Ahmad, and J. Menezes. 2001. Epstein-Barr virus and its glycoprotein-350 upregulate IL-6 in human B cells via CD21, involving activation of NF-kB and different signaling pathways. J. Mol. Biol. 308:501-514. - PubMed
-
- Fearon, D. T., and R. H. Carter. 1995. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu. Rev. Immunol. 13:127-149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

