The critical role of voltage-dependent calcium channel in axonal repair following mechanical trauma
- PMID: 17448606
- PMCID: PMC2701192
- DOI: 10.1016/j.neuroscience.2007.02.015
The critical role of voltage-dependent calcium channel in axonal repair following mechanical trauma
Abstract
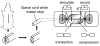
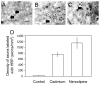
Membrane disruption following mechanical injury likely plays a critical role in the pathology of spinal cord trauma. It is known that intracellular calcium is a key factor that is essential to membrane resealing. However, the differential role of calcium influx through the injury site and through voltage dependent calcium channels (VDCC) has not been examined in detail. Using a well-established ex vivo guinea-pig spinal cord white matter preparation, we have found that axonal membrane resealing was significantly inhibited following transection or compression in the presence of cadmium, a non-specific calcium channel blocker, or nimodipine, a specific L-type calcium channel blocker. Membrane resealing was assessed by the changes of membrane potential and compound action potential (CAP), and exclusion of horseradish peroxidase 60 min following trauma. Furthermore, 1 microM BayK 8644, a VDCC agonist, significantly enhanced membrane resealing. Interestingly, this effect was completely abolished when the concentration of BayK 8644 was increased to 30 microM. These data suggest that VDCC play a critical role in membrane resealing. Further, there is likely an appropriate range of calcium influx through VDCC which ensures effective axonal membrane resealing. Since elevated intracellular calcium has also been linked to axonal deterioration, blockage of VDCC is proposed to be a clinical treatment for various injuries. The knowledge gained in this study will likely help us better understand the role of calcium in various CNS trauma, which is critical for designing new approaches or perhaps optimizing the effectiveness of existing methods in the treatment of CNS trauma.
Figures
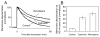

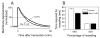

Similar articles
-
Polyethylene glycol enhances axolemmal resealing following transection in cultured cells and in ex vivo spinal cord.J Neurotrauma. 2010 Jan;27(1):151-61. doi: 10.1089/neu.2009.0993. J Neurotrauma. 2010. PMID: 19691421
-
Spinal cord regeneration induced by a voltage-gated calcium channel agonist.Neurol Res. 2002 Oct;24(7):639-42. doi: 10.1179/016164102101200672. Neurol Res. 2002. PMID: 12392197
-
Dimethylsulfoxide enhances CNS neuronal plasma membrane resealing after injury in low temperature or low calcium.J Neurocytol. 2001 Sep-Oct;30(9-10):829-39. doi: 10.1023/a:1019645505848. J Neurocytol. 2001. PMID: 12165673
-
Voltage-dependent calcium channels regulate GH4 pituitary cell proliferation at two stages of the cell cycle.J Cell Physiol. 1991 Feb;146(2):197-206. doi: 10.1002/jcp.1041460203. J Cell Physiol. 1991. PMID: 1705563
-
Demyelination in spinal cord injury and multiple sclerosis: what can we do to enhance functional recovery?J Neurotrauma. 1992 Mar;9 Suppl 1:S105-17. J Neurotrauma. 1992. PMID: 1588601 Review.
Cited by
-
Cellular mechanisms and signals that coordinate plasma membrane repair.Cell Mol Life Sci. 2018 Oct;75(20):3751-3770. doi: 10.1007/s00018-018-2888-7. Epub 2018 Jul 26. Cell Mol Life Sci. 2018. PMID: 30051163 Free PMC article. Review.
-
Trauma-induced plasmalemma disruptions in three-dimensional neural cultures are dependent on strain modality and rate.J Neurotrauma. 2011 Nov;28(11):2219-33. doi: 10.1089/neu.2011.1841. J Neurotrauma. 2011. PMID: 22023556 Free PMC article.
-
Therapeutic approaches for spinal cord injury.Clinics (Sao Paulo). 2012 Oct;67(10):1219-24. doi: 10.6061/clinics/2012(10)16. Clinics (Sao Paulo). 2012. PMID: 23070351 Free PMC article. Review.
-
Diffuse traumatic axonal injury in the optic nerve does not elicit retinal ganglion cell loss.J Neuropathol Exp Neurol. 2013 Aug;72(8):768-81. doi: 10.1097/NEN.0b013e31829d8d9d. J Neuropathol Exp Neurol. 2013. PMID: 23860030 Free PMC article.
-
Roles of channels and receptors in the growth cone during PNS axonal regeneration.Exp Neurol. 2010 May;223(1):38-44. doi: 10.1016/j.expneurol.2009.10.001. Epub 2009 Oct 13. Exp Neurol. 2010. PMID: 19833126 Free PMC article. Review.
References
-
- Agrawal SK, Nashmi R, Fehlings MG. Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience. 2000;99:179–188. - PubMed
-
- Borgens RB, Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000;14:27–35. - PubMed
-
- Cano-Abad MF, Villarroya M, Garcia AG, Gabilan NH, Lopez MG. Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J Biol Chem. 2001;276:39695–39704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

