Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence
- PMID: 17445891
- PMCID: PMC1955686
- DOI: 10.1016/j.neubiorev.2007.02.004
Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence
Abstract
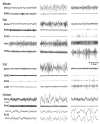
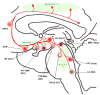
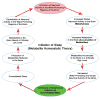
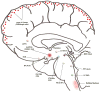
At its most basic level, the function of mammalian sleep can be described as a restorative process of the brain and body; recently, however, progressive research has revealed a host of vital functions to which sleep is essential. Although many excellent reviews on sleep behavior have been published, none have incorporated contemporary studies examining the molecular mechanisms that govern the various stages of sleep. Utilizing a holistic approach, this review is focused on the basic mechanisms involved in the transition from wakefulness, initiation of sleep and the subsequent generation of slow-wave sleep and rapid eye movement (REM) sleep. Additionally, using recent molecular studies and experimental evidence that provides a direct link to sleep as a behavior, we have developed a new model, the cellular-molecular-network model, explaining the mechanisms responsible for regulating REM sleep. By analyzing the fundamental neurobiological mechanisms responsible for the generation and maintenance of sleep-wake behavior in mammals, we intend to provide a broader understanding of our present knowledge in the field of sleep research.
Figures

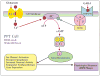
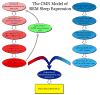
Similar articles
-
Neural Circuitry of Wakefulness and Sleep.Neuron. 2017 Feb 22;93(4):747-765. doi: 10.1016/j.neuron.2017.01.014. Neuron. 2017. PMID: 28231463 Free PMC article. Review.
-
Suprachiasmatic nucleus in sleep-wake regulation.Sleep Med. 2007 Dec;8 Suppl 3:27-33. doi: 10.1016/j.sleep.2007.10.003. Sleep Med. 2007. PMID: 18032104 Review.
-
A quartet neural system model orchestrating sleep and wakefulness mechanisms.J Neurophysiol. 2006 Apr;95(4):2055-69. doi: 10.1152/jn.00575.2005. Epub 2005 Nov 9. J Neurophysiol. 2006. PMID: 16282204
-
Mechanisms of sleep-wake cycle modulation.CNS Neurol Disord Drug Targets. 2009 Aug;8(4):245-53. doi: 10.2174/187152709788921654. CNS Neurol Disord Drug Targets. 2009. PMID: 19689306 Review.
-
[Selective stimulations and lesions of the rat brain nuclei as the models for research of the human sleep pathology mechanisms].Glas Srp Akad Nauka Med. 2011;(51):85-97. Glas Srp Akad Nauka Med. 2011. PMID: 22165729 Review. Serbian.
Cited by
-
Orexinergic bouton density is lower in the cerebral cortex of cetaceans compared to artiodactyls.J Chem Neuroanat. 2015 Oct;68:61-76. doi: 10.1016/j.jchemneu.2015.07.007. Epub 2015 Jul 30. J Chem Neuroanat. 2015. PMID: 26232521 Free PMC article.
-
Local use-dependent sleep.Curr Top Med Chem. 2011;11(19):2390-1. doi: 10.2174/156802611797470295. Curr Top Med Chem. 2011. PMID: 22181666 Free PMC article. No abstract available.
-
A novel role for calcium/calmodulin kinase II within the brainstem pedunculopontine tegmentum for the regulation of wakefulness and rapid eye movement sleep.J Neurochem. 2010 Jan;112(1):271-81. doi: 10.1111/j.1471-4159.2009.06452.x. Epub 2009 Oct 26. J Neurochem. 2010. PMID: 19860859 Free PMC article.
-
Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence.J Neurochem. 2010 Nov;115(3):782-94. doi: 10.1111/j.1471-4159.2010.06980.x. Epub 2010 Sep 28. J Neurochem. 2010. PMID: 20807311 Free PMC article.
-
Cellular and chemical neuroscience of mammalian sleep.Sleep Med. 2010 May;11(5):431-40. doi: 10.1016/j.sleep.2010.02.002. Epub 2010 Mar 31. Sleep Med. 2010. PMID: 20359944 Free PMC article. Review.
References
-
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. - PubMed
-
- Aghajanian GK, Wang RY, Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–175. - PubMed
-
- Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, Szymusiak R. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–216. - PubMed
-
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in rapid eye movement sleep. Am J Physiol. 1995;269:R1250–1257. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

