The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings
- PMID: 17429075
- PMCID: PMC1877118
- DOI: 10.1091/mbc.e07-02-0154
The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings
Abstract
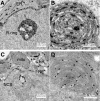
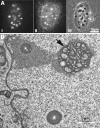
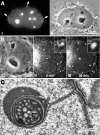
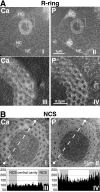
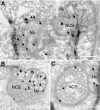
The nucleolar channel system (NCS) is a well-established ultrastructural hallmark of the postovulation endometrium. Its transient presence has been associated with human fertility. Nevertheless, the biogenesis, composition, and function of these intranuclear membrane cisternae are unknown. Membrane systems with a striking ultrastructural resemblance to the NCS, termed R-rings, are induced in nuclei of tissue culture cells by overexpression of the central repeat domain of the nucleolar protein Nopp140. Here we provide a first molecular characterization of the NCS and compare the biogenesis of these two enigmatic organelles. Like the R-rings, the NCS consists of endoplasmic reticulum harboring the marker glucose-6-phosphatase. R-ring formation initiates at the nuclear envelope, apparently by a calcium-mediated Nopp140-membrane interaction, as supported by the calcium-binding ability of Nopp140, the inhibition of R-ring formation by calcium chelators, and the concentration of Nopp140 and complexed calcium in R-rings. Although biogenesis of the NCS may initiate similarly, the reduced presence of complexed calcium and Nopp140 suggests the involvement of additional factors.
Figures

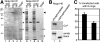
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
-
Progesterone Threshold Determines Nucleolar Channel System Formation in Human Endometrium.Reprod Sci. 2014 Jul;21(7):915-920. doi: 10.1177/1933719113519177. Epub 2014 Jan 23. Reprod Sci. 2014. PMID: 24458483 Free PMC article.
-
The Novel Nuclear Envelope Protein KAKU4 Modulates Nuclear Morphology in Arabidopsis.Plant Cell. 2014 May;26(5):2143-2155. doi: 10.1105/tpc.113.122168. Epub 2014 May 13. Plant Cell. 2014. PMID: 24824484 Free PMC article.
-
Nopp140-mediated concentration of telomerase in Cajal bodies regulates telomere length.Mol Biol Cell. 2019 Dec 15;30(26):3136-3150. doi: 10.1091/mbc.E19-08-0429. Epub 2019 Oct 30. Mol Biol Cell. 2019. PMID: 31664887 Free PMC article.
-
Nuclear Import of the HIV-1 Core Precedes Reverse Transcription and Uncoating.Cell Rep. 2020 Sep 29;32(13):108201. doi: 10.1016/j.celrep.2020.108201. Cell Rep. 2020. PMID: 32997983 Free PMC article.
-
Extruded Nucleoli of Human Dental Pulp Cells.Medicina (Kaunas). 2022 Feb 10;58(2):260. doi: 10.3390/medicina58020260. Medicina (Kaunas). 2022. PMID: 35208583 Free PMC article.
References
-
- Areas J. A., Grobner G., Glaubitz C., Watts A. Interaction of a type II myosin with biological membranes studied by 2H solid state NMR. Biochemistry. 1998;37:5582–5588. - PubMed
-
- Azadian-Boulanger G., Secchi J., Laraque F., Raynaud J. P., Sakiz E. Action of midcycle contraceptive (R 2323) on the human endometrium. Am. J. Obstet. Gynecol. 1976;125:1049–1056. - PubMed
-
- Bentin-Ley U., Sjogren A., Nilsson L., Hamberger L., Larsen J. F., Horn T. Presence of uterine pinopodes at the embryo-endometrial interface during human implantation in vitro. Hum. Reprod. 1999;14:515–520. - PubMed
-
- Chiu C. M., Tsay Y. G., Chang C. J., Lee S. C. Nopp140 is a mediator of the protein kinase A signaling pathway that activates the acute phase response alpha1-acid glycoprotein gene. J. Biol. Chem. 2002;277:39102–39111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

