High glucose induces IL-1beta expression in human monocytes: mechanistic insights
- PMID: 17426109
- PMCID: PMC2676171
- DOI: 10.1152/ajpendo.00718.2006
High glucose induces IL-1beta expression in human monocytes: mechanistic insights
Abstract
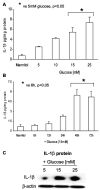
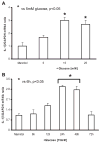
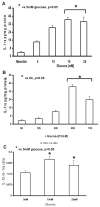
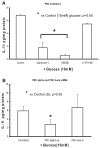
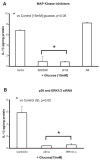
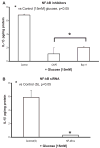
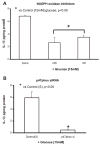
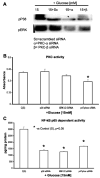
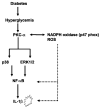
Previously, IL-1beta secretion from Type 2 diabetic patients has been shown to be increased compared with controls. In this study, we aimed to delineate the mechanism of IL-1beta induction under high-glucose (HG) conditions in human monocytes. THP-1 cells cultured in normal glucose were treated with increasing concentrations of d-glucose (10-25 mM) for 6-72 h. IL-1beta and IL-1 receptor antagonist levels were measured by ELISA and Western blots, whereas mRNA was quantitated by RT-PCR. Specific inhibitors and small interfering RNAs of PKC, p38, ERK1/2, NF-kappaB, and NADPH oxidase were used to determine the mediators in parallel experiments under HG conditions. IL-1beta-secreted protein, cellular protein, and mRNA increase under HG conditions is time and dose dependent, with maximum increase at 15 mM (48 h; P < 0.05). IL-1 receptor antagonist release was time and dose dependent, similar to IL-1beta expression pattern; however, the molar ratio of IL-1beta to IL-1RA was increased. Data from inhibitor and small interfering RNA experiments indicate that IL-1beta release under HG is mediated by PKC-alpha, via phosphorylation of p38 MAPK, and ERK1/2 leading to NF-kappaB activation, resulting in increased mRNA and protein for IL-1beta. At the same time, it appears that NADPH oxidase via p47phox activates NF-kappaB, resulting in increased IL-1beta secretion. Data suggest that, under HG conditions, monocytes release significantly higher amounts of IL-1beta through multiple mechanisms, further compounding the disease progression. Targeting signaling pathways mediating IL-1beta release could result in the amelioration of inflammation and possibly diabetic vasculopathies.
Figures

Similar articles
-
Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors.Am J Physiol Endocrinol Metab. 2011 Jan;300(1):E145-54. doi: 10.1152/ajpendo.00490.2010. Epub 2010 Oct 19. Am J Physiol Endocrinol Metab. 2011. PMID: 20959532 Free PMC article.
-
PAF effects on MCP-1 and IL-6 secretion in U-937 monocytes in comparison with oxLDL and IL-1β effects.Atherosclerosis. 2011 Dec;219(2):519-25. doi: 10.1016/j.atherosclerosis.2011.07.123. Epub 2011 Aug 5. Atherosclerosis. 2011. PMID: 21920519
-
Exogenous H2S protects H9c2 cardiac cells against high glucose-induced injury and inflammation by inhibiting the activation of the NF-κB and IL-1β pathways.Int J Mol Med. 2015 Jan;35(1):177-86. doi: 10.3892/ijmm.2014.2007. Epub 2014 Nov 19. Int J Mol Med. 2015. PMID: 25412187
-
Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}.Diabetes. 2005 Jan;54(1):85-91. doi: 10.2337/diabetes.54.1.85. Diabetes. 2005. PMID: 15616014
-
The unconventional secretion of IL-1β: Handling a dangerous weapon to optimize inflammatory responses.Semin Cell Dev Biol. 2018 Nov;83:12-21. doi: 10.1016/j.semcdb.2018.03.011. Epub 2018 Apr 5. Semin Cell Dev Biol. 2018. PMID: 29571971 Review.
Cited by
-
Associations between obesity-related gene expression in maternal and cord blood and newborn adiposity: findings from the Araraquara Cohort study.Int J Obes (Lond). 2021 Sep;45(9):1958-1966. doi: 10.1038/s41366-021-00857-8. Epub 2021 May 17. Int J Obes (Lond). 2021. PMID: 34002037
-
Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study.Diabetes. 2010 May;59(5):1222-7. doi: 10.2337/db09-1199. Epub 2010 Feb 25. Diabetes. 2010. PMID: 20185814 Free PMC article.
-
Anti-hyperglycemic contours of Madhugrit are robustly translated in the Caenorhabditis elegans model of lipid accumulation by regulating oxidative stress and inflammatory response.Front Endocrinol (Lausanne). 2022 Dec 5;13:1064532. doi: 10.3389/fendo.2022.1064532. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36545334 Free PMC article.
-
Tubular IL-1β Induces Salt Sensitivity in Diabetes by Activating Renal Macrophages.Circ Res. 2022 Jun 24;131(1):59-73. doi: 10.1161/CIRCRESAHA.121.320239. Epub 2022 May 16. Circ Res. 2022. PMID: 35574842 Free PMC article.
-
New Insights into the Pivotal Role of Iron/Heme Metabolism in TLR4/NF-κB Signaling-Mediated Inflammatory Responses in Human Monocytes.Cells. 2021 Sep 27;10(10):2549. doi: 10.3390/cells10102549. Cells. 2021. PMID: 34685529 Free PMC article.
References
-
- Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P. Glucose ingestion induces an increase in intranuclear NF-κB, a fall in cellular inhibitor κB, and an increase in TNF-alpha mRNA by mononuclear cells in healthy human subjects. Metabolism. 2006;55:1177–1185. - PubMed
-
- Aljada A, Ghanim H, Dandona P. Translocation of p47phox and activation of NADPH oxidase in mononuclear cells. Methods Mol Biol. 2002;196:99–103. - PubMed
-
- Asakawa H, Miyagawa J, Hanafusa T, Kuwajima M, Matsuzawa Y. High glucose and hyperosmolarity increase secretion of interleukin-1β incultured human aortic endothelial cells. J Diabetes Complications. 1997;11:176–179. - PubMed
-
- Baeuerle PA. The inducible transcription activator NF-κB: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

