Photodynamic therapy-generated tumor cell lysates with CpG-oligodeoxynucleotide enhance immunotherapy efficacy in human papillomavirus 16 (E6/E7) immortalized tumor cells
- PMID: 17425690
- PMCID: PMC11159296
- DOI: 10.1111/j.1349-7006.2007.00447.x
Photodynamic therapy-generated tumor cell lysates with CpG-oligodeoxynucleotide enhance immunotherapy efficacy in human papillomavirus 16 (E6/E7) immortalized tumor cells
Abstract
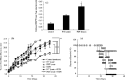
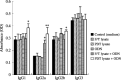
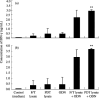
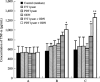
Immunotherapy with photodynamic therapy (PDT) offers great promise as a new alternative for cancer treatment; however, its use remains experimental. In this study, we examined the immunotherapeutic significance of human papillomavirus (HPV)-immortalized tumor cell lysates induced by PDT with CpG-oligodeoxynucleotide (ODN). PDT-cell lysates were generated by irradiating Radachlorin (5 microg/mL) preloaded TC-1 cells carrying HPV 16 E7. PDT-cell lysates plus ODN coinjection for protection against E7-expressing tumors as well as specific immune responses were evaluated with the following tests: heat shock protein 70 (HSP70) enzyme-linked immunosorbent assay, in vitro and in vivo tumor growth inhibition, interferon-gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha) assay, cytotoxic T-lymphocyte assay, and fluorescence activated cell sorting (FACS) analysis. PDT-cell lysates plus ODN coinjection showed a significant suppression of tumor growth at both prophylactic and therapeutic levels, compared to PDT (or F/T)-cell lysates or ODN alone. In addition, we evaluated the level of the immune response with the coinjection. HSP70, an important regulator of inflammatory and immune response, was observed in abundance in the PDT-cell lysates. IFN-gamma production and cytotoxic T lymphocytes (CTL) responses were induced by PDT-cell lysates plus ODN injection. The coinjection resulted in PDT-cell lysate-specific antibodies (IgG1, IgG2a, IgG2b, and IgG3) and T-helper cell responses significantly higher than PDT-cell lysates alone. Moreover, IFN-gamma production and CTL responses were significantly induced in the PDT-cell lysate plus ODN immunized groups. These enhanced immune responses appeared to be mediated by CD8+ T cells only. These data suggest that PDT-cell lysates plus ODN injection may be an effective approach to induce CTL immune responses as a possible immunotherapeutic strategy for cancer therapy.
Figures

Similar articles
-
Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8+ T cells in protection.Cancer Res. 2002 Dec 15;62(24):7234-40. Cancer Res. 2002. PMID: 12499264
-
Photodynamic therapy with recombinant adenovirus AdmIL-12 enhances anti-tumour therapy efficacy in human papillomavirus 16 (E6/E7) infected tumour model.Immunology. 2008 Aug;124(4):461-8. doi: 10.1111/j.1365-2567.2007.02797.x. Epub 2008 Apr 4. Immunology. 2008. PMID: 18397271 Free PMC article.
-
Mannose-Modified Liposome Co-Delivery of Human Papillomavirus Type 16 E7 Peptide and CpG Oligodeoxynucleotide Adjuvant Enhances Antitumor Activity Against Established Large TC-1 Grafted Tumors in Mice.Int J Nanomedicine. 2020 Dec 1;15:9571-9586. doi: 10.2147/IJN.S275670. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33293808 Free PMC article.
-
CpG-ODN-stimulated dendritic cells act as a potent adjuvant for E7 protein delivery to induce antigen-specific antitumour immunity in a HPV 16 E7-associated animal tumour model.Immunology. 2004 May;112(1):117-25. doi: 10.1111/j.1365-2567.2004.01851.x. Immunology. 2004. PMID: 15096191 Free PMC article.
-
The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire.Photochem Photobiol. 2019 Nov;95(6):1288-1305. doi: 10.1111/php.13173. Epub 2019 Nov 10. Photochem Photobiol. 2019. PMID: 31602649 Free PMC article. Review.
Cited by
-
CpG-conjugated apoptotic tumor cells elicit potent tumor-specific immunity.Cancer Immunol Immunother. 2011 May;60(5):659-69. doi: 10.1007/s00262-011-0973-y. Epub 2011 Feb 4. Cancer Immunol Immunother. 2011. PMID: 21318638 Free PMC article.
-
Enhancing cancer immunotherapy with photodynamic therapy and nanoparticle: making tumor microenvironment hotter to make immunotherapeutic work better.Front Immunol. 2024 Apr 5;15:1375767. doi: 10.3389/fimmu.2024.1375767. eCollection 2024. Front Immunol. 2024. PMID: 38646546 Free PMC article. Review.
-
CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer.J Biophotonics. 2014 Nov;7(11-12):897-905. doi: 10.1002/jbio.201300072. Epub 2013 Aug 7. J Biophotonics. 2014. PMID: 23922221 Free PMC article.
-
Therapeutic effects of systemic photodynamic therapy in a leukemia animal model using A20 cells.Lasers Med Sci. 2012 Mar;27(2):445-52. doi: 10.1007/s10103-011-0950-x. Epub 2011 Jul 18. Lasers Med Sci. 2012. PMID: 21769639
-
Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles.ACS Nano. 2014 Jun 24;8(6):5670-81. doi: 10.1021/nn5002112. Epub 2014 May 13. ACS Nano. 2014. PMID: 24801008 Free PMC article.
References
-
- Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg 2002; 20: 3–7. - PubMed
-
- Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol 2001; 74: 656–69. - PubMed
-
- Yuan J, Mahama‐Relue PA, Fournier RL, Hampton JA. Predictions of mathematical models of tissue oxygenation and generation of singlet oxygen during photodynamic therapy. Radiat Res 1997; 148: 386–94. - PubMed
-
- Korbelik M. Role of Toll‐like receptors in photodynamic therapy‐elicited host response. Proc SPIE 2004; 5319: 87–95.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

