Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans
- PMID: 17404618
- PMCID: PMC1838941
- DOI: 10.1172/JCI30328
Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans
Abstract
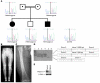
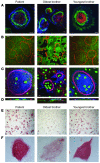
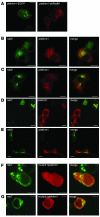
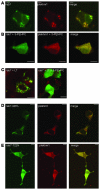
This study illustrates that Plekhm1 is an essential protein for bone resorption, as loss-of-function mutations were found to underlie the osteopetrotic phenotype of the incisors absent rat as well as an intermediate type of human osteopetrosis. Electron and confocal microscopic analysis demonstrated that monocytes from a patient homozygous for the mutation differentiated into osteoclasts normally, but when cultured on dentine discs, the osteoclasts failed to form ruffled borders and showed little evidence of bone resorption. The presence of both RUN and pleckstrin homology domains suggests that Plekhm1 may be linked to small GTPase signaling. We found that Plekhm1 colocalized with Rab7 to late endosomal/lysosomal vesicles in HEK293 and osteoclast-like cells, an effect that was dependent on the prenylation of Rab7. In conclusion, we believe PLEKHM1 to be a novel gene implicated in the development of osteopetrosis, with a putative critical function in vesicular transport in the osteoclast.
Figures



Similar articles
-
A new heterozygous mutation (R714C) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (PLEKHM1), impairs vesicular acidification and increases TRACP secretion in osteoclasts.J Bone Miner Res. 2008 Mar;23(3):380-91. doi: 10.1359/jbmr.071107. J Bone Miner Res. 2008. PMID: 17997709
-
Characterization of a Relatively Malignant Form of Osteopetrosis Caused by a Novel Mutation in the PLEKHM1 Gene.J Bone Miner Res. 2016 Nov;31(11):1979-1987. doi: 10.1002/jbmr.2885. Epub 2016 Jul 13. J Bone Miner Res. 2016. PMID: 27291868
-
TRAFD1 (FLN29) Interacts with Plekhm1 and Regulates Osteoclast Acidification and Resorption.PLoS One. 2015 May 19;10(5):e0127537. doi: 10.1371/journal.pone.0127537. eCollection 2015. PLoS One. 2015. PMID: 25992615 Free PMC article.
-
Advances in osteoclast biology resulting from the study of osteopetrotic mutations.Hum Genet. 2009 Jan;124(6):561-77. doi: 10.1007/s00439-008-0583-8. Epub 2008 Nov 6. Hum Genet. 2009. PMID: 18987890 Review.
-
Recent developments in the understanding of the pathophysiology of osteopetrosis.Eur J Endocrinol. 1996 Feb;134(2):143-56. doi: 10.1530/eje.0.1340143. Eur J Endocrinol. 1996. PMID: 8630510 Review.
Cited by
-
Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain.Mol Biol Cell. 2010 Dec;21(23):4162-72. doi: 10.1091/mbc.E10-06-0495. Epub 2010 Oct 13. Mol Biol Cell. 2010. PMID: 20943950 Free PMC article.
-
New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis.Osteoporos Int. 2011 Jan;22(1):1-20. doi: 10.1007/s00198-010-1272-8. Epub 2010 May 11. Osteoporos Int. 2011. PMID: 20458572 Review.
-
A Rab33b missense mouse model for Smith-McCort dysplasia shows bone resorption defects and altered protein glycosylation.Front Genet. 2023 Jun 8;14:1204296. doi: 10.3389/fgene.2023.1204296. eCollection 2023. Front Genet. 2023. PMID: 37359363 Free PMC article.
-
Sorting Nexin 10 as a Key Regulator of Membrane Trafficking in Bone-Resorbing Osteoclasts: Lessons Learned From Osteopetrosis.Front Cell Dev Biol. 2021 May 20;9:671210. doi: 10.3389/fcell.2021.671210. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095139 Free PMC article.
-
Bone-cartilage crosstalk informed by aging mouse bone transcriptomics and human osteoarthritis genome-wide association studies.Bone Rep. 2022 Dec 13;18:101647. doi: 10.1016/j.bonr.2022.101647. eCollection 2023 Jun. Bone Rep. 2022. PMID: 36636109 Free PMC article.
References
-
- Balemans W., Van Wesenbeeck L., Van Hul W. A clinical and molecular overview of the human osteopetroses. Calcif. Tissue Int. 2005;77:263–274. - PubMed
-
- Sly W.S., Hewett-Emmett D., Whyte M.P., Yu Y.S., Tashian R.E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc. Natl. Acad. Sci. U. S. A. 1983;80:2752–2756. - PMC - PubMed
-
- Kornak U., et al. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum. Mol. Genet. 2000;9:2059–2063. - PubMed
-
- Frattini A., et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 2000;25:343–346. - PubMed
-
- Kornak U., et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

