CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand
- PMID: 17404306
- PMCID: PMC4402709
- DOI: 10.4049/jimmunol.178.8.5227
CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand
Abstract
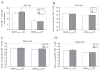
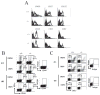
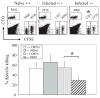
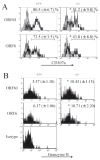
Studies of costimulatory receptors belonging to the TNFR family have revealed their diverse roles in affecting different stages of the T cell response. The 4-1BB ligand (4-1BBL)/4-1BB pathway has emerged as a receptor-ligand pair that impacts not the initial priming, but later phases of the T cell response, such as sustaining clonal expansion and survival, maintaining memory CD8(+) T cells, and supporting secondary expansion upon Ag challenge. Although the role of this costimulatory pathway in CD8(+) T cell responses to acute viral infections has been well-studied, its role in controlling chronic viral infections in vivo is not known to date. Using the murine gammaherpesvirus-68 (MHV-68) model, we show that 4-1BBL-deficient mice lack control of MHV-68 during latency and show significantly increased latent viral loads. In contrast to acute influenza infection, the numbers of MHV-68-specific memory CD8(+) T cells were maintained during latency. However, the virus-specific CD8(+) T cells showed defects in function, including decreased cytolytic function and impaired secondary expansion. Thus, 4-1BBL deficiency significantly affects the function, but not the number, of virus-specific CD8(+) T cells during gammaherpesvirus latency, and its absence results in an increased viral burden. Our study suggests that the 4-1BB costimulatory pathway plays an important role in controlling chronic viral infections.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures



Similar articles
-
A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo.J Immunol. 2004 Jan 15;172(2):981-8. doi: 10.4049/jimmunol.172.2.981. J Immunol. 2004. PMID: 14707071
-
4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy.J Immunol. 2001 Aug 1;167(3):1313-24. doi: 10.4049/jimmunol.167.3.1313. J Immunol. 2001. PMID: 11466348
-
Gammaherpesvirus latency differentially impacts the generation of primary versus secondary memory CD8+ T cells during subsequent infection.J Virol. 2014 Nov;88(21):12740-51. doi: 10.1128/JVI.02106-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142586 Free PMC article.
-
Exploiting 4-1BB costimulation for enhancing antiviral vaccination.Viral Immunol. 2006 Winter;19(4):593-601. doi: 10.1089/vim.2006.19.593. Viral Immunol. 2006. PMID: 17201654 Review.
-
Immune regulation by 4-1BB and 4-1BBL: complexities and challenges.Immunol Rev. 2009 May;229(1):192-215. doi: 10.1111/j.1600-065X.2009.00765.x. Immunol Rev. 2009. PMID: 19426223 Review.
Cited by
-
CD8(+) T cells from mice transnuclear for a TCR that recognizes a single H-2K(b)-restricted MHV68 epitope derived from gB-ORF8 help control infection.Cell Rep. 2012 May 31;1(5):461-71. doi: 10.1016/j.celrep.2012.03.009. Epub 2012 Apr 26. Cell Rep. 2012. PMID: 22832272 Free PMC article.
-
T-cell costimulatory blockade in organ transplantation.Cold Spring Harb Perspect Med. 2013 Dec 1;3(12):a015537. doi: 10.1101/cshperspect.a015537. Cold Spring Harb Perspect Med. 2013. PMID: 24296352 Free PMC article. Review.
-
Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response.J Immunol. 2008 Jan 15;180(2):1148-57. doi: 10.4049/jimmunol.180.2.1148. J Immunol. 2008. PMID: 18178855 Free PMC article.
-
CD137 Plays Both Pathogenic and Protective Roles in Type 1 Diabetes Development in NOD Mice.J Immunol. 2017 May 15;198(10):3857-3868. doi: 10.4049/jimmunol.1601851. Epub 2017 Mar 31. J Immunol. 2017. PMID: 28363905 Free PMC article.
-
CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help.J Immunol. 2012 Apr 15;188(8):3829-38. doi: 10.4049/jimmunol.1103329. Epub 2012 Mar 14. J Immunol. 2012. PMID: 22422886 Free PMC article.
References
-
- Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Semin Immunol. 2004;16:185–196. - PubMed
-
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
-
- Whitmire JK, Ahmed R. Costimulation in antiviral immunity: differential requirements for CD4+ and CD8+ T cell responses. Curr Opin Immunol. 2000;12:448–455. - PubMed
-
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

