Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior
- PMID: 17392475
- PMCID: PMC6672130
- DOI: 10.1523/JNEUROSCI.4749-06.2007
Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior
Abstract
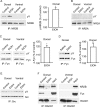
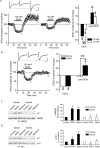
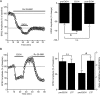
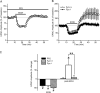
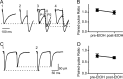
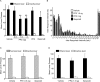
Addiction is characterized by compulsive alcohol or drug taking and seeking, and the dorsal striatum has been implicated in such maladaptive persistent habits. The NMDA receptor (NMDAR), which is a major target of alcohol, is implicated in striatal-based habit learning. We found that, in the dorsal striatum, alcohol (ethanol) exposure produced an increase in the phosphorylation of the NR2B subunit of the NMDAR, and a corresponding increase in the activity of Fyn kinase, which phosphorylates NR2B. We further observed an ethanol-mediated long-term facilitation (LTF) of the activity of NR2B-containing NMDARs (NR2B-NMDARs) in the dorsal striatum. This LTF is Fyn kinase dependent, because it was observed in Fyn wild-type but not in Fyn knock-out mice. Importantly, none of these biochemical and physiological changes was observed in the ventral striatum. Finally, dorsal but not ventral striatum infusion of a Fyn or NR2B-NMDAR inhibitor reduced rat operant self-administration of ethanol. Our results suggest that the Fyn-mediated phosphorylation and LTF of NR2B-NMDAR activity in the dorsal striatum after exposure to ethanol may underlie aberrant plasticity that contributes to mechanisms underlying alcohol drinking behavior.
Figures
Similar articles
-
Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse.J Neurosci. 2010 Jul 28;30(30):10187-98. doi: 10.1523/JNEUROSCI.2268-10.2010. J Neurosci. 2010. PMID: 20668202 Free PMC article.
-
Ethanol-mediated long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum.Channels (Austin). 2011 May-Jun;5(3):205-9. doi: 10.4161/chan.5.3.14856. Epub 2011 May 1. Channels (Austin). 2011. PMID: 21289476 Free PMC article.
-
Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior.J Neurosci. 2012 Oct 24;32(43):15124-32. doi: 10.1523/JNEUROSCI.2783-12.2012. J Neurosci. 2012. PMID: 23100433 Free PMC article.
-
[Regulation of NMDA receptor function by Fyn-mediated tyrosine phosphorylation].Nihon Shinkei Seishin Yakurigaku Zasshi. 2002 Oct;22(5):165-7. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002. PMID: 12451687 Review. Japanese.
-
Regulation of NMDA receptors by the tyrosine kinase Fyn.FEBS J. 2012 Jan;279(1):12-9. doi: 10.1111/j.1742-4658.2011.08391.x. Epub 2011 Dec 5. FEBS J. 2012. PMID: 21985328 Review.
Cited by
-
Ethanol-induced increases in extracellular dopamine are blunted in brain-derived neurotrophic factor heterozygous mice.Neurosci Lett. 2011 Feb 11;489(3):172-6. doi: 10.1016/j.neulet.2010.12.010. Epub 2010 Dec 14. Neurosci Lett. 2011. PMID: 21163332 Free PMC article.
-
Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase.Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6650-5. doi: 10.1073/pnas.1017856108. Epub 2011 Apr 4. Proc Natl Acad Sci U S A. 2011. PMID: 21464302 Free PMC article.
-
GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8114-9. doi: 10.1073/pnas.0711755105. Epub 2008 Jun 9. Proc Natl Acad Sci U S A. 2008. PMID: 18541917 Free PMC article.
-
Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence.Neurosci Biobehav Rev. 2012 Feb;36(2):873-88. doi: 10.1016/j.neubiorev.2011.11.002. Epub 2011 Nov 11. Neurosci Biobehav Rev. 2012. PMID: 22101113 Free PMC article. Review.
-
Contribution of an SFK-Mediated Signaling Pathway in the Dorsal Hippocampus to Cocaine-Memory Reconsolidation in Rats.Neuropsychopharmacology. 2016 Feb;41(3):675-85. doi: 10.1038/npp.2015.217. Epub 2015 Jul 23. Neuropsychopharmacology. 2016. PMID: 26202103 Free PMC article.
References
-
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. - PubMed
-
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

