Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins
- PMID: 17389686
- PMCID: PMC1863825
- DOI: 10.1242/jcs.03436
Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins
Abstract
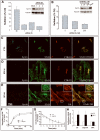
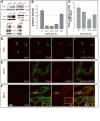
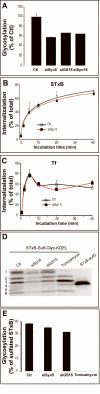
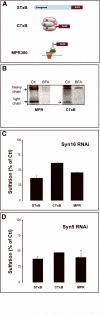
Retrograde transport allows proteins and lipids to leave the endocytic pathway to reach other intracellular compartments, such as trans-Golgi network (TGN)/Golgi membranes, the endoplasmic reticulum and, in some instances, the cytosol. Here, we have used RNA interference against the SNARE proteins syntaxin 5 and syntaxin 16, combined with recently developed quantitative trafficking assays, morphological approaches and cell intoxication analysis to show that these SNARE proteins are not only required for efficient retrograde transport of Shiga toxin, but also for that of an endogenous cargo protein - the mannose 6-phosphate receptor - and for the productive trafficking into cells of cholera toxin and ricin. We have found that the function of syntaxin 16 was specifically required for, and restricted to, the retrograde pathway. Strikingly, syntaxin 5 RNA interference protected cells particularly strongly against Shiga toxin. Since our trafficking analysis showed that apart from inhibiting retrograde endosome-to-TGN transport, the silencing of syntaxin 5 had no additional effect on Shiga toxin endocytosis or trafficking from TGN/Golgi membranes to the endoplasmic reticulum, we hypothesize that syntaxin 5 also has trafficking-independent functions. In summary, our data demonstrate that several cellular and exogenous cargo proteins use elements of the same SNARE machinery for efficient retrograde transport between early/recycling endosomes and TGN/Golgi membranes.
Figures
Similar articles
-
The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport.J Cell Biol. 2011 Aug 8;194(3):459-72. doi: 10.1083/jcb.201102045. Epub 2011 Aug 1. J Cell Biol. 2011. PMID: 21807881 Free PMC article.
-
The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network.Mol Biol Cell. 2007 Dec;18(12):4979-91. doi: 10.1091/mbc.e07-06-0622. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914056 Free PMC article.
-
Trans-Golgi network syntaxin 10 functions distinctly from syntaxins 6 and 16.Mol Membr Biol. 2005 Jul-Aug;22(4):313-25. doi: 10.1080/09687860500143829. Mol Membr Biol. 2005. PMID: 16154903
-
Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6.Biosci Rep. 2012 Aug;32(4):383-91. doi: 10.1042/BSR20120006. Biosci Rep. 2012. PMID: 22489884 Free PMC article. Review.
-
Syntaxin 16's Newly Deciphered Roles in Autophagy.Cells. 2019 Dec 17;8(12):1655. doi: 10.3390/cells8121655. Cells. 2019. PMID: 31861136 Free PMC article. Review.
Cited by
-
Lack of the endosomal SNAREs vti1a and vti1b led to significant impairments in neuronal development.Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2575-80. doi: 10.1073/pnas.1013891108. Epub 2011 Jan 24. Proc Natl Acad Sci U S A. 2011. PMID: 21262811 Free PMC article.
-
Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis.Nature. 2015 Jan 22;517(7535):493-6. doi: 10.1038/nature14064. Epub 2014 Dec 17. Nature. 2015. PMID: 25517096 Free PMC article.
-
Yeast Reporter Assay to Identify Cellular Components of Ricin Toxin A Chain Trafficking.Toxins (Basel). 2016 Dec 6;8(12):366. doi: 10.3390/toxins8120366. Toxins (Basel). 2016. PMID: 27929418 Free PMC article.
-
A Conserved Structural Motif Mediates Retrograde Trafficking of Shiga Toxin Types 1 and 2.Traffic. 2015 Dec;16(12):1270-87. doi: 10.1111/tra.12338. Epub 2015 Nov 2. Traffic. 2015. PMID: 26420131 Free PMC article.
-
The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport.J Cell Biol. 2011 Aug 8;194(3):459-72. doi: 10.1083/jcb.201102045. Epub 2011 Aug 1. J Cell Biol. 2011. PMID: 21807881 Free PMC article.
References
-
- Amessou M, Popoff V, Yelamos B, Saint-Pol A, Johannes L. Recent methods for studying retrograde transport. Curr. Protocols Cell. Biol. in press. - PubMed
-
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. - PubMed
-
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 1996;271:17961–17965. - PubMed
-
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2001;2:98–106. - PubMed
-
- Dascher C, Matteson J, Balch WE. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J. Biol. Chem. 1994;269:29363–29366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

