Human immunopathogenesis of severe acute respiratory syndrome (SARS)
- PMID: 17374415
- PMCID: PMC7114310
- DOI: 10.1016/j.virusres.2007.02.014
Human immunopathogenesis of severe acute respiratory syndrome (SARS)
Abstract
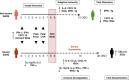
Progressive immune-associated injury is a hallmark of severe acute respiratory syndrome (SARS). Viral evasion of innate immunity, hypercytokinemia and systemic immunopathology in the SARS coronavirus (SARS CoV) infected host have been suggested as possible mechanisms for the cause of severe pathology and morbidity in SARS patients. The molecular and cellular basis for how SARS CoV impacts the host immune system resulting in severe SARS, however, has not been elucidated. The variable clinical course of SARS may be the result of complex programs of host responses against the infectious agent. Therefore, the systematic analysis of innate and adaptive immune responses to SARS CoV is imperative in building as complete an immunological model as possible of host immunity and inflammatory responses during illness. Here we review recent advances in SARS immunopathogenesis research and present a summary of our findings regarding host responses in SARS patients. We contend that dysregulated type I and II interferon (IFN) responses during SARS may culminate in a failure of the switch from hyper-innate immunity to protective adaptive immune responses in the human host.
Figures

Similar articles
-
Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome.J Virol. 2007 Aug;81(16):8692-706. doi: 10.1128/JVI.00527-07. Epub 2007 May 30. J Virol. 2007. PMID: 17537853 Free PMC article.
-
Lack of innate interferon responses during SARS coronavirus infection in a vaccination and reinfection ferret model.PLoS One. 2012;7(9):e45842. doi: 10.1371/journal.pone.0045842. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029269 Free PMC article.
-
Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis.mBio. 2018 Oct 9;9(5):e01753-18. doi: 10.1128/mBio.01753-18. mBio. 2018. PMID: 30301856 Free PMC article.
-
Pathogenesis of severe acute respiratory syndrome.Curr Opin Immunol. 2005 Aug;17(4):404-10. doi: 10.1016/j.coi.2005.05.009. Curr Opin Immunol. 2005. PMID: 15950449 Free PMC article. Review.
-
SARS coronavirus and innate immunity.Virus Res. 2008 Apr;133(1):101-12. doi: 10.1016/j.virusres.2007.03.015. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451827 Free PMC article. Review.
Cited by
-
An update on emerging therapeutics to combat COVID-19.Basic Clin Pharmacol Toxicol. 2021 Aug;129(2):104-129. doi: 10.1111/bcpt.13600. Epub 2021 Jun 11. Basic Clin Pharmacol Toxicol. 2021. PMID: 33977663 Free PMC article. Review.
-
Systemic innate and adaptive immune responses to SARS-CoV-2 as it relates to other coronaviruses.Hum Vaccin Immunother. 2020 Dec 1;16(12):2980-2991. doi: 10.1080/21645515.2020.1802974. Epub 2020 Sep 2. Hum Vaccin Immunother. 2020. PMID: 32878546 Free PMC article. Review.
-
The Swine IFN System in Viral Infections: Major Advances and Translational Prospects.Pathogens. 2022 Jan 27;11(2):175. doi: 10.3390/pathogens11020175. Pathogens. 2022. PMID: 35215119 Free PMC article. Review.
-
Recently discovered human coronaviruses.Clin Lab Med. 2009 Dec;29(4):715-24. doi: 10.1016/j.cll.2009.07.007. Clin Lab Med. 2009. PMID: 19892230 Free PMC article. Review.
-
Calming the cytokine storm of COVID-19 through inhibition of JAK2/STAT3 signaling.Drug Discov Today. 2022 Feb;27(2):390-400. doi: 10.1016/j.drudis.2021.10.016. Epub 2021 Oct 28. Drug Discov Today. 2022. PMID: 34743903 Free PMC article. Review.
References
-
- Antonio G.E., Ooi C.G., Wong K.T., Tsui E.L., Wong J.S., Sy A.N., Hui J.Y., Chan C.Y., Huang H.Y., Chan Y.F., Wong T.P., Leong L.L., Chan J.C., Ahuja A.T. Radiographic-clinical correlation in severe acute respiratory syndrome: study of 1373 patients in Hong Kong. Radiology. 2005;237:1081–1090. - PubMed
-
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., Ephtimios I.E., Kitai L., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W., Poutanen S.M., Detsky A.S. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J. Am. Med. Assoc. 2003;289:2801–2809. - PubMed
-
- Bosinger S.E., Hosiawa K.A., Cameron M.J., Persad D., Ran L., Xu L., Boulassel M.R., Parenteau M., Fournier J., Rud E.W., Kelvin D.J. Gene expression profiling of host response in models of acute HIV infection. J. Immunol. 2004;173:6858–6863. - PubMed
-
- Cameron, M.J., Gold, W., Ran, L., Poutanen, S., Louie, M., Phillips, E., Mederski, B., Loutfy, M., Mazzulli, T., Willey, B., Muller, M., Persad, D., Xu, L., Brunton, J., Low, D., McGeer, A., Kelvin, D., 2003. Identification of gene expression profiles in patients with severe acute respiratory syndrome (SARS) that may be predictive of diagnosis, severity and clinical outcome of the illness. [Abstr. 43rd Intersci Conf Antimicrob Agents Chemother, Abstract no. K-452j].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

