P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells
- PMID: 17351655
- PMCID: PMC2012976
- DOI: 10.1038/sj.bjp.0707213
P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells
Abstract
Background and purpose: The pro-inflammatory cytokine, interleukin-1beta (IL-1beta), has been implicated in the pathogenesis of atherosclerosis, potentially via its release from vascular endothelium. Endothelial cells (EC) synthesize IL-1beta in response to inflammatory stimuli, but the demonstration and mechanism of release of IL-1 from ECs remains unclear. In activated monocytes, efficient release of bioactive IL-1beta occurred via activation of ATP-gated P2X(7) receptors (P2X(7)Rs). Activation of P2X(7)R in ECs from human umbilical vein (HUVECs) released IL-1 receptor antagonist (IL-1Ra). The purpose of this study was to provide a quantitative investigation of P2XR expression and function, in parallel with IL-1beta and IL-1Ra synthesis, processing and release, in HUVECs under pro-inflammatory conditions.
Experimental approach: Quantitative RT-PCR, immunoblotting, ELISA, flow cytometry, and whole-cell patch clamp recordings were used to determine protein expression and receptor function. IL-8-luciferase-reporter was used as an IL-1 sensitive bioassay.
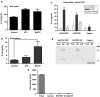
Key results: HUVECs expressed P2X(4)R and P2X(7)R subtypes and both were significantly up-regulated under inflammatory conditions. P2X(7)R currents were increased 3-fold by inflammatory stimuli, whereas no P2X(4)R-mediated currents were detected. Caspase-1, but not IL-1beta, was present intracellularly under basal conditions; inflammatory stimuli activated the synthesis of intracellular pro-IL-1beta and increased caspase-1 levels. Activation of P2X(7)Rs resulted in low-level release of bioactive IL-1beta and simultaneous release of IL-1Ra. The net biological effect of release was anti-inflammatory.
Conclusions and implications: Endothelial P2X(7)Rs induced secretion of both pro- and anti-inflammatory IL-1 receptor ligands, the balance of which may provide a means for altering the inflammatory state of the arterial vessel wall.
Figures

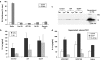
Similar articles
-
Characterization of ATP-gated P2X7 receptors in fish provides new insights into the mechanism of release of the leaderless cytokine interleukin-1 beta.Mol Immunol. 2007 Feb;44(6):1286-99. doi: 10.1016/j.molimm.2006.05.015. Epub 2006 Jul 11. Mol Immunol. 2007. PMID: 16837047
-
Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation.J Immunol. 2004 Jul 15;173(2):1202-8. doi: 10.4049/jimmunol.173.2.1202. J Immunol. 2004. PMID: 15240711
-
Purinergic P2X7 receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival.Diabetologia. 2009 Aug;52(8):1579-88. doi: 10.1007/s00125-009-1349-0. Epub 2009 Apr 25. Diabetologia. 2009. PMID: 19396427 Free PMC article.
-
Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells.Crit Rev Immunol. 2009;29(4):335-45. doi: 10.1615/critrevimmunol.v29.i4.40. Crit Rev Immunol. 2009. PMID: 19673687 Review.
-
The P2X7 receptor: a key player in IL-1 processing and release.J Immunol. 2006 Apr 1;176(7):3877-83. doi: 10.4049/jimmunol.176.7.3877. J Immunol. 2006. PMID: 16547218 Review.
Cited by
-
Docosahexaenoic acid reduces adenosine triphosphate-induced calcium influx via inhibition of store-operated calcium channels and enhances baseline endothelial nitric oxide synthase phosphorylation in human endothelial cells.Korean J Physiol Pharmacol. 2019 Sep;23(5):345-356. doi: 10.4196/kjpp.2019.23.5.345. Epub 2019 Aug 26. Korean J Physiol Pharmacol. 2019. PMID: 31496872 Free PMC article.
-
Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells.Br J Pharmacol. 2009 Aug;157(7):1215-24. doi: 10.1111/j.1476-5381.2009.00287.x. Epub 2009 Jun 22. Br J Pharmacol. 2009. PMID: 19552691 Free PMC article.
-
Purinergic signaling: A gatekeeper of blood-brain barrier permeation.Front Pharmacol. 2023 Feb 7;14:1112758. doi: 10.3389/fphar.2023.1112758. eCollection 2023. Front Pharmacol. 2023. PMID: 36825149 Free PMC article. Review.
-
Magnesium sulfate inhibits inflammation through P2X7 receptors in human umbilical vein endothelial cells.Pediatr Res. 2020 Feb;87(3):463-471. doi: 10.1038/s41390-019-0557-7. Epub 2019 Sep 7. Pediatr Res. 2020. PMID: 31493768 Free PMC article.
-
Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations.J Biol Chem. 2011 Apr 1;286(13):11067-81. doi: 10.1074/jbc.M110.198879. Epub 2011 Jan 24. J Biol Chem. 2011. PMID: 21262970 Free PMC article.
References
-
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. - PubMed
-
- Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. - PubMed
-
- Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, et al. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

